Statistical Analysis of Clinical Variants of Wilson Disease Among Males and Females in Local Population of Wah Cantt, Pakistan
| Received 10 Apr, 2024 |
Accepted 02 May, 2024 |
Published 07 Jun, 2024 |
The study aimed to identify the clinical variables of Wilson's disease (WD) among males and females in the local population. Wilson disease is an autosomal recessive inherited disorder that causes copper (Cu) accumulation in the body's liver, brain, and other vital organs. This research is being conducted to check the association of various symptoms of WD among males and females. A sample of 49 patients was obtained from different hospitals in Wah Cantt, Punjab, Pakistan. Among those, 29 were male and 20 were female. Frequency and percentage were computed for qualitative variables, while we used the mean and standard deviation to describe quantitative variables. The chi-square technique was used to check the significance of clinical variants of Wilson's disease among males and females. Maximum respondents, ages ranging from 16 to 20 years, were categorized into intervals. Among females, the frequency percentage was greater than that of males. In the population where parental cousin marriage (PCM) existed, there was a lower frequency percentage compared to those where consanguinity didn't exist, and the calculated p-value demonstrates that the results are significant at the 1% level. Yellow sclera had a higher frequency percentage in males than females, so the calculated p-value indicated that the results were significant at the 1% level. The prevalence of chronic liver disease was higher in men as compared to women. The calculated p-value indicated that the results are significant at 5%. Antinuclear antibodies don't exist among males and females, and the computed p-value indicated that they don't exist. The Keyser-Fischer (KF) ring presence was observed in a higher ratio in females than males, and the calculated p-value suggested that a relationship does not exist. The percentage of the absence of the symptom Spider Nivai was 100% among males and females, as it is not as recurring in Wilson's disease.
INTRODUCTION
Copper (Cu) is present in various plant and animal foods, and the average human diet typically provides approximately 1400 for men and 1100 mcg/day for women. While the accumulation of Cu in plants and seafood increases due to discharge from industries without proper man clinical presentation is related to hepatic and neurological disturbances. Six metal binding domains (MBD) present at the N-terminal are also required to transport Cu (Plauth et al., 2006). The most common mutation in European countries is a point mutation H1069Q in exon 14, but this mutation is not found in Asian countries (Plauth et al., 2006). Mutational data from Asian countries is mainly available from Pakistan, China, Japan, India and South Korea. Ceruloplasmin moves in the human body via an endocytotic vesicle towards canaliculus of bile to maintain the Cu homeostasis in normal individuals (Arredondo & Núñez 2005; Pnagement, it also increases in humans due to the intake of such types of food and causes severe diseases like Wilson disease. Wilson disease is an autosomal recessive inherited disorder that causes copper accumulation in the liver, brain and other vital organs like kidneys and lungs (Mitra & Metcalf, 2009; Brewer et al., 1992). In 1912, Kinner Wilson was the first to describe it as a progressive lenticular degeneration disorder. The role of copper in (WD) was recognized in the 1990s, but the gene responsible for this disorder was not found until 1993. The cause of the disease is a failure to excrete unneeded and excessive Cu in the bile for loss in the stool. This may be due to a failure to excrete Cu packaged in ceruloplasmin into the bile (Brewer et al., 1992). More than 600 mutations in the ATP7B gene have been documented up till now from various countries, according to WD database (Gupta et al., 2013). Of all 21 exons, exons 2, 8, 13, 14, 16,18 and 21 are considered mutations hotspot exons. The most common mutation in European countries is a point mutation H1069Q in exon 14, but this mutation is not found in Asian countries (Plauth et al., 2006). A mutation in the ATP7B gene is located on chromosome 13q14.3. It is a large gene that possesses 21 exons, has a size of 80kb, and is responsible for Wilson's disease (Tarrier et al.,1967). Wilson's disease is multi-symptomatic, but the most commoiel et al., 2013). Acute temporary hepatitis is the mode of presentation in 25% of those in whom hepatic symptoms herald disease onset. However, hemolytic anemia associated with hepatic dysfunction or increment of unconjugated (indirect) bilirubin should alert the clinician to the possibility of Wilson's disease. The hepatic symptoms of Wilson's disease may also mimic autoimmune hepatitis. In this setting, ceruloplasmin, as an acute-phase reactant, may rise transiently into the low normal range. Children affected by the disease may present with hepatomegaly or changes in enzyme concentration, such as ceruloplasmin and aminotransferases. Liver cirrhosis with hypertension in the portal region, along with splenomegaly, is an important clinical sign in patients with chronic liver failure (Korman et al., 2008). In the case of consanguinity (parental cousin marriage), the rate of homozygous mutations may increase and ultimately lead to WD likelihood. In individuals with Wilson's disease, a mutation in the ATP7B gene results in a defective ATP7B protein, which cannot perform these functions properly (Hung et al.,1997). As a result, Cu gradually accumulates within the liver cells. Not only does this gradual Cu accumulation trade-off the hepatic function and its storage capacity, but it also eventually tends to spill out exceeded and unbound Cu of the liver and is deposited in other organs (brain) and tissues, which can cause damage and dysfunction. As extra Cu is released from the liver, the urinary Cu excretion rises dramatically, but it cannot fully compensate for the defect in biliary excretion (Parker & Picut 2005). However, it was suggested that a reduction in the protein, X-linked inhibitor of apoptosis (XIAP), induced by Cu elevation, accelerates caspase 3–3-initiated apoptosis with resultant cell death (Mufti et al., 2006). A golden-greenish granular layer is usually visible to the naked eye (de Bie et al., 2007). It exists in patients with neurological and psychiatric involvement of WD than in young patients with hepatic abnormalities (Höglinger et al.,2017). On liver transplantation, it is not eradicated; the chances of recrudescence are always there. Elevated Cu content in WD may reach a level greater than 250 μg/g dry weight. It is considered the most accurate biochemical evidence for establishing a diagnosis. Due to sampling errors in measuring hepatic parenchymal Cu concentration, the amount of Cu may not be calculated properly, which might give a false negative result. The basal ganglia region of the brain is examined for changes, but in some cases, the central pons and tectal plate regions have also shown abnormalities (Pasricha et al., 2010). In very few patients, a 'giant panda face' is reported in scans, considered a characteristic feature of WD (Davis & Garrity, 2007; Piel et al., 2013). In the late stages of the disease, secondary brain atrophy occurs. Glucose movement in the basal ganglia region of the brain also decreases in WD. Still, this finding is very nonspecific and cannot be used to differentiate WD from other neurodegenerative disorders (Hedera, 2017). Because of the variability in Wilson's disease manifestation, the diagnosis of the disease cannot be confirmed unequivocally by using a single protocol. Different diagnostic approaches summarized in Table 1 are combined to confirm WD in patients, making diagnosis complicated for physicians.
Neurological disorders
Neurological abnormalities can be categorized as ataxia, akinetic rigid syndrome, dystonia, and pseudosclerosis (Taly et al., 2007). Dystonia, motor neuron impairments in the skull region, drooling, an open jaw, and mucus production are essential symptoms of neurological abnormalities (del Rosario et al., 1998). It may cause involuntary head rotation, eye closure by force, loss of speech, difficulty swallowing, abnormal neck posturing, an inability to move, and immobility (Svetel et al., 2001). In early neurologic disease, a person may lose concentration and ability to coordinate; headaches, insomnia, and migraines are also observed, but seizures are not that frequent. Tremors are reported in 22 to 25 percent of patients diagnosed with the disease. Patients showing neurological symptoms of the disease may or may not have significant hepatic abnormalities (Brewer et al.,1992). Psychiatric and behavioural symptoms are very nonspecific, so they are often regarded as problems associated with puberty. Children with Wilson's disease show poor performance at school, mood swings, and inappropriate behaviour (Ferenci, 2004). Abnormal posture and cramping of different parts of the body occur in cases of dystonia syndrome. It is reported in 10 to 65 percent of all patients with neurologic dysfunction. It may cause involuntary head rotation, eye closure by force, loss of speech, difficulty swallowing, abnormal neck posturing, and inability to move, and the patient may become bedridden (Svetel et al., 2001).
Ophthalmological manifestation
Kayser-Fleisher ring (KF ring) is a golden-brown ring in 95 percent of patients suffering from WD who have increased Cu concentrations in their bodies. In 85 percent of patients reported to have an abnormal neurological condition, a KF ring is found (Birkholz & Oetting, 2009). An experienced ophthalmologist examines the eye in suspected patients for WD to detect the KF ring and establish a diagnosis. Kayser-Fleischer rings are formed by the deposition of copper within Descemet's membrane (Fig. 1). Excess Cu is deposited throughout the Cornea in Wilson's disease, but only in Descemet's membrane are Sulphur-copper complexes formed, producing the visible copper deposits (Wiebers et al., 1977).
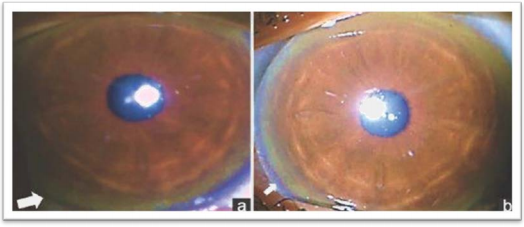
|
The prevalence of KF ring is 33% to 65% in individuals with hepatic involvement in the disease. Pre-symptomatic individuals may also develop KF ring in 40% of cases, but due to initiation of treatment using chelating agents, it will disappear in 85 percent cases (Roberts & Schilsky 2008; Birkholz & Oetting, 2009). Less frequent disease symptoms include gigantism, osteoarthritis, vertebral column abnormalities, hemolytic anemia, osteoporosis, pancreatitis, glucose intolerance, cardiomyopathy and myopathy. In females, repeated miscarriage, delayed puberty, menstrual irregularities and infertility are also associated with Wilson's disease.
| Table 1: | Scoring system for Wilson's disease developed at the 8th International Meeting, Leipzig 2001 (Ferenci et al. 2003) | |||
| Common signs and clinical symptoms | Score |
| Kayser-Fleischer ring (KF ring) | |
| Present | 2 |
| Absent | 0 |
| Serum ceruloplasmin | |
| Normal (>0.2 g/L) | 0 |
| 0.1-0.2 g/L | 1 |
| <0.1 g/L | 2 |
| Neurological symptoms | |
| Severe | 2 |
| Mild | 1 |
| Absent | 0 |
| Coombs negative hemolytic anemia | |
| Present | 1 |
| Absent | 0 |
| Other tests | |
| Liver copper (in the absence of cholestasis) | |
| >5x ULN* (>4 μmol/g) | 2 |
| 0.8-4 μmol/g | 1 |
| Normal (<0.8 μmol/g) | -1 |
| Rhodanine-positive granules | 1 |
| Urinary copper (in the absence of acute hepatitis) | |
| Normal | 0 |
| 1-2x ULN | 1 |
| >2x ULN | 2 |
| Normal, but >5x ULN after D- penicillamine | 2 |
| Mutational analysis | |
| On both chromosomes detected | 4 |
| on 1 chromosome detected | 1 |
| No mutations detected | 0 |
| Total score: | Evaluation |
| 4 or more | Diagnosis established |
| 3 | Diagnosis possible, more tests needed |
| 2 or less | Diagnosis very unlikely |
Magnetic resonance imaging (MRI)
Patients having neuropsychiatric manifestations of WD are evaluated by magnetic resonance imaging (MRI) and computerized tomography (CT) scan for abnormalities in the brain (Fig. 2).
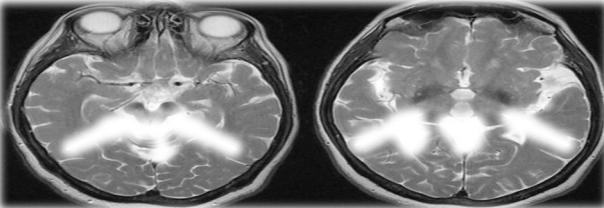
|
During the measurement of hepatic parenchymal Cu concentration, due to sampling errors, the amount of Cu may not be adequately calculated and may give a false negative result. In heterozygotes, the hepatic Cu concentration is higher than in homozygous normal individuals, but it is always higher. Primarily, the basal ganglia region of the brain is examined for changes, but in some cases, the central pons and tectal-plate region have also shown abnormalities (Namestnikova et al.,2017; Prashanth et al., 2010; Ogra & Suzuki, 1998). The discharge of heavy metals like Cu in the environment threatens the edible crops that people ultimately use as a meal, disturbs the human metabolism, and results in human liver disorder. The main objectives of the present studies are to examine Wilson's disease patients in the local population of Wahcantt, Pakistan, with a focus on the demographic characteristics of the participants and the association of risk factors for Wilson's disease with gender in that area.
MATERIAL AND METHODS
Study design
A prospective cross-sectional study design is used in the present study. Clinical and laboratory data were collected from consecutive Wilson's disease (WD) patients. The evaluation included a detailed physical examination, conventional laboratory testing, genetic analysis, and a liver biopsy. The descriptive, exploratory, and non-contrived study was designed to investigate the association of various symptoms of Wilson's disease among males and females. This study was generally prospective, and 49 patients' demographics were obtained over six months. Patients were asked to fill out demographic information along with an instrument measuring the symptoms, causes, and effects of WD. A questionnaire was used to collect information regarding their disease and associated symptoms. Patients were assured that their data would be kept confidential. Participants in the study were approached from different hospitals in Wah Cantt and Islamabad. The study consisted of 49 participants; 20 (40.8%) were female, and 29 (59.2%) were male. The age ranges of participants were 6–20 years. For data collection, participants were informed about the study's purpose. Consent to participate in the study was acquired from them after providing verbal instruction.
Statistical analysis of Non-Genetic Risk factors
Information was utilized to check the association of risk factors of WD with gender among variables (age, age of onset, gender, blood group, ethnicity, area, PCM, family history, bilirubin, total bilirubin, education, urinary copper 24/hrs) using SPSS 25.0.
Identification and Enrollment of patients affected with WD
The study was carried out from August 2022 to February 2023. 49 patients affected by WD were involved in this study. Detailed information about the disease and a demographic profile on a specific Performa were collected from diseased and healthy controls.
Sample study
49 patients (male and female) were selected from different hospitals in Wah Cantt and Islamabad who showed the signs and symptoms of Wilson's disease. Among them, 28 patients were reported from POF Wah Cantt, and 21 patients (male and female) were reported from the hospitals in Islamabad by local references, making the total sample size approximately 49.
Pilot testing
Permission was obtained from the University of WAH for pilot testing through a permission letter issued by the University. The task was improved after pilot testing.
Data Collection
Samples of patients affected by WD were collected by using a questionnaire and ensuring the patients that the information would be kept confidential.
Study and Procedure
The study's design was descriptive, and it was a questionnaire survey. The following procedure was followed for the collection of data:
Data was collected from the patients in hospitals for six months, from August 2022 to February 2023, by showing the permission letter given by the University. The questionnaire was distributed to a sample of patients in the hospital. Patients were given a brief introduction to the content of the questionnaire. It was thoroughly explained to the patients. The data was collected personally with the help of a questionnaire. The researcher visited all the hospitals personally to collect data. Every patient took an average of about 15 minutes to respond. Patients were not allowed to discuss during the completion of the questionnaire. At the end, participants were thanked for their cooperation.
Data Analysis
After data collection, the data processing and analysis are done using SPSS 25.0. The frequencies and percentages of the demographic profile of the sample are computed. To check the significance of different factors related to Wilson's disease among males and females, a Chi-square test is used that independently compares the variables to determine whether they are related. The chi-square test is a statistical test used to compare observed results with expected results, and it is also used to study the relationship among variables. The Chi-square statistic appears as an option when requesting a cross-tabulation in SPSS, and is used in a test of independence labelled Pearson Chi-square. A statistic can be evaluated by comparing the actual value against a critical value found in the Chi-square distribution. It depends upon the p-value calculated to demonstrate whether variables are dependent or independent and whether there is a statistical relationship between the categorical variables (Tu & Blackwell 1967; Gibbs & Walshe 1979).
RESULTS AND DISCUSSION
The aim of the present research was the statistical analysis of clinical variables of Wilson's disease among males and females in a local population to study the symptoms, causes, and effects of Wilson's disease (WD) among males and females. The impact of the factors is computed across demographics. After data collection, processing and analysis are made using SPSS 25.0. The frequencies and percentages of the demographic profile of the sample are computed. A chi-square test checks the significance of factors related to Wilson's disease (WD) among males and females (Table 2).
| Table 2: | Demographic Characteristics of Surveyed Pa participants (=) | |||
| Variables | Categories Mean ± SD | (% )15.6±2.03 |
| Age (Years) | 6-10 | 2 (4.1) |
| 11-15 | 14 (28.6) | |
| 16-20 | 33 (67.3) | |
| Mean ± SD | 13.5±2.24 | |
| Age at onset (years) | 6-10 | 7 (14.3) |
| 11-15 | 36 (73.5) | |
| 16-20 | 6 (12.2) | |
| Gender | Females | 20 (40.8) |
| Males | 29 (59.2) | |
| Blood group | A+ | 6(12.2) |
| A- | 3(6.1) | |
| B+ | 23(46.9) | |
| B- | 10(20.4) | |
| O+ | 5(10.2) | |
| O- | 2(4.1) | |
| Ethnicity | Punjabi | 21(42.9) |
| Sindhi | 18(36.7) | |
| Kpk | 1(2.0) | |
| Balochistan | 9(18.4) | |
| Area | Urban | 24(49.0) |
| Rural | 25(51.0) | |
| Education | Matric | 36(73.5) |
| Middle | 10(20.4) | |
| FSc | 3(6.1) | |
| PCM | Yes | 24(49.0) |
| No | 25(51.0) | |
| Family history | Yes | 8(16.3) |
| No | 41(83.7) | |
| Bilirubin u/molL | Mean ± 3SD | 3.69±3(11.96) |
| Total bilirubin umol/L | Mean ± 3SD | 11.96±3 (6.38) |
| Urinary copper/24 hrs | Mean ± 3SD | 117.1±3(191.4) |
The frequency (Table 3) reveals that the mean±3SD age is 15.6±3 (2.03) years. Also, it is observed that the maximum number of respondents (67.3%) ages are between 16 and 20 years, and the ages categorized in intervals 6 to 10 and 11 to 15 years are 4.1% and 28.6%, respectively. Ages at the onset of the disease are categorized in intervals of (14.3%), (73.5%), and (12.2%), respectively. The frequency percentage was 40.8% in females and 59.2% in males (Eghtesad et al., 1999). With respect to blood groups categorized as A+, A-, B+, B-, O+, and O- having percentages (12.2%), (6.1%), (46.9%), (20.4), (10.2), and (4.10), respectively, ethnicity was checked at parameters regarding Punjabi (42.9%), Sindhi (36.7%), KPK (2.0%), and Balochistan (18.4%). The percentage frequency was calculated at 51.0% among the rural population and 49.0% among the urban population. Education was categorized into subgroups: middle (20.4%), matric (73.5%), and FSC (6.1%). In the population where parental cousin marriage exists, the percentage was calculated at 49.0%; among those where consanguinity doesn't exist, the percentage was 51.0%. For individuals having in their family history, the existence frequency percentage was (16.3%) and (83.7%).
| Table 3: | Association of Risk Factors of WD with Gender ( =) | |||
| Variables/Category | Total | Male (%) | Females (%) | P-Value (%) |
| Total | 49 | 29 (59.2) | 20 (40.8) | |
| -100 | ||||
| PCM | 0.000 | |||
| Yes | 24 (49.0) | 8 (27.6) | 16 (80.0) | |
| No | 25 (51.0) | 21 (72.4) | 4 (20.0) | |
| Family History | 0.445 | |||
| Yes | 8(16.3) | 6(20.7) | 2(10.0) | |
| No | 41(83.7) | 23(79.3) | 18(19.0) | |
| Yellow sclera | .000 | |||
| Yes | 33(67.3) | 29(100.0 | 4(20.0) | |
| No | 16 (32.7) | 0(0.00) | 16(80.0) | |
| Fatigue | 0.001 | |||
| Yes | 11(22.4) | 11(37.9) | 0(0.00) | |
| No | 38 (77.6) | 18(62.1) | 20(100.0) | |
| Anoxia | 0.135 | |||
| Yes | 4(8.2) | 4(13.8) | 0(0.00) | |
| No | 45(91.8) | 25(86.2) | 20(100.0) | |
| Psychiatric | 0.001 | |||
| Yes | 12(24.5) | 12(41.4) | 0(0.0) | |
| No | 45(91.8) | 17(58.6) | 20(100.0) | |
| Confusion | 0.069 | |||
| Yes | 6(12.2) | 6(20.7) | 0(0.0) | |
| No | 45(91.8) | 23(79.3) | 20(100) | |
| Jaundice | .000 | |||
| Yes | 28(57.1) | 26(89.7) | 2(10.0) | |
| No | 37(75.5) | 3(10.3) | 18(90.0) | |
| Lower limb edema | 0.002 | |||
| Yes | 18(36.7) | 16(55.2) | 2(10.0) | |
| No | 31(63.3) | 13(44.8) | 18(90.0) | |
| Spider Nivai | ||||
| Yes | - | - | - | |
| No | 49(100.0) | 29(100.0) | 21(100) | |
| Abnormal movements | 0.001 | |||
| Yes | 12(24.5) | 12(41.4) | 0(0.00) | |
| No | 37(75.5) | 17(58.6) | 20(100) | |
| Chronic liver disease | 0.008 | |||
| Yes | 44(89.8) | 29(100) | 15(75.0) | |
| No | 5(10.2) | 0(0.00) | 5(25.0) | |
| Antinuclear antibody | ||||
| Yes | 0 (0.0) | NA | NA | |
| No | 49 | 29 | 20 (100.0 | |
| -100 | -100 | |||
| Anti smooth antibody | 0.003 | |||
| Yes | 36(73.5) | 17(58.6) | 19(95.0) | |
| No | 13(26.5) | 12(41.4) | 1(5.0) | |
| Hepatitis b antigen | 0.062 | |||
| Yes | 3(6.1) | 0(0.00) | 3(15.0) | |
| No | 46(93.9) | 29(100.0) | 17(85.0) | |
| Hepatitis c antigen | ||||
| Yes | - | - | - | |
| No | 49(100.0) | 29(100.0) | 21(100.0) | |
| Fibrosis stage 2-4 | 0.015 | |||
| Yes | 31(63.3) | 14(48.3) | 17(85.0) | |
| No | 18(36.7) | 15(51.7) | 3(15.0) | |
| Copper quantification | 0.408 | |||
| Normal | 1(2.0) | 0(0.00) | 1(5.0) | |
| Abnormal | 48(98.0) | 29(100.0) | 19(95.0) | |
| Level of liver copper | 0.000 | |||
| Normal | 21(42.9) | 3(10.3) | 18(90.0) | |
| Abnormal | 28(57.1) | 26(89.7) | 2(10.0) | |
| Kf rings | 0.646 |
Table (3) describes the association of risk factors of Wilson's disease with gender. In a total population of 49 individuals, 59.2%were males and 40.8% were females, and parental cousin marriage existed 49.0% among males 27.6% and females80.0% whereas. Among those where parental cousin marriage does not exist, the total percentage was calculated at 51.0% among males, 72.4%, and females at 20.0%. The p-value was calculated as 0.000, showing that the result is significant at the 1% level. In an association of gender with family history, the total percentage among existed population was 16.3%, of which males were 16.3%and 20.7% in females. In contrast, the absence of family history was noted in 83.7% of males, 79.3%, and in females19.0%. The p-value is calculated as 0.445, meaning the results are significant at a 5% level. Among the patients where the symptom of yellow sclera appeared in total frequency was 67.3%, to whom males 100.0% and females 20.0%, the absence of such symptom did not appear in total percentages 32.7% among them 0.00% and 80.0% .The p-value calculated as 000 means that results are significant at 1% level. The symptoms of fatigue existed in 22.4% of the population, of which 37.9% were males and 0.000% were females. The symptoms did not appear in 77.6%, of which 2.1% were males, and 100.0 were females. The p-value indicates that the results are significant at 1% level. Anoxia appeared 8.2%population, of which 13.8% were males and 0.00% were females. The absence of such symptoms was recorded in 91.8%, among which males were 86.2% and females were 100.0%. The p-value is 0.135, indicating that the results are significant at 10%. Psychiatric symptoms appeared in a population of 24.5%, of which 41.4% were males, and 0.00% were females, and the absence of symptoms was recorded in 91.8% of the population, of which 58.6% were males and 100.0% were females (Chun et al, 2012). The p-value indicates the result is significant at a 1% level calculated as 0.001. Symptoms of confusion were present in 12.2% of the population. Among them, 20.7% were male, and 0.00% were women to whom symptoms appeared; however, 91.8% were the population in which symptoms did not appear, 79.3% were men and 100.0% were females. The p-value, as calculated, is 0.69, which is not significant at 10%, which means the relation does not exist. The jaundice was present in 57.1% of patients; 89.7% were males, and 10.0% were females. The absence of symptoms was recorded in 75.5% of patients. Among them, 10.3% were males and 90.0% were females. The p-value indicates 0.00, meaning that the results are significant at 1%. Pregnancy management in women with Wilson disease (WD) remains an important clinical problem (Xu et al., 2019). Lower limb edema was present in 36.7% of the patients; 55.2% were males, and 10.0% were females. The absence of such symptoms was recorded in 63.3% of the population. Among the females, 90.0% were males, and 44.8% were males. The p-value indicates the results are insignificant; thus, no relation exists. The percentage of the un-existence of a symptom of spider Nivai was 100.0% in both males and females, so it is not significant in Wilson's disease (Schilsky et al.1989). The abnormal movement was 24.5% in patients, 41.4% in males and 0.00% in females. Such a symptom was not recorded in 75.5% of the population, of which 58.6% were males and 100.0% were females. The p-value demonstrates 0.01, indicating that the results are significant at 5% level. Chronic liver disease has appeared in 89.9% of the population; among them, 100.0% were men, and 75.0% were female; in those where the symptom did not exist, having the frequency 10.2% involved, 100.0% were male, and 25.0% were female. The p-value of 0.00 means the result is significant at 5% (Rudnick & Perlmutter 2005). Antinuclear antibodies were not present among males and females; they were absent in 100.0% of the population. The p-value indicates that the results are insignificant and a relation does not exist, similar to the case of the hepatitis C antigen, which is not present among the males and females in a population of 49 individuals. Hepatitis B antigen was present in 3.6% of the population; 0.00% were male and 15.0% were female. This symptom was absent in 93.9% of the population; 100.0% were male and 85.0% were female. The p-value was calculated as 06, indicating that the results are insignificant and a relationship does not exist. Fibrosis stage 2–4 existed. 63.3% of the population, 48.3% were males and 85.0% were females. The symptoms did not appear in 36.7% of the population, of which 51.7% were males and 15.0% were females. The p-value is 0.01, indicating the result is significant at the 1% level. Cu quantification was normal in 2.0% of the population, of which 0.00% were males and 5.0% were females. The abnormal level of Cu quantification existed in 98.0% of the population, of which 100.0% were males and 95.0% were females. The p-value of 0.40 indicates that the results are significant at the 5% level. The level of liver Cu was normal in 42.9% of the population; males were 10.3%, and females population percentage was 90.0%. The abnormal quantification in the liver was observed in 57.1% of the population; of those, 89.7% were males and 10.0% were females. The calculated p-value was 0.00, indicating the results are significant at the 1% level. KF rings were present in 71.4% of the population, of which 69.0% were males and 75.0% were females. The absence of KF rings was observed in 28.6% of individuals, among whom 31.0% were males, and a p-value of 25.0% was calculated as 0.64, indicating that a relationship does not exist.
Wilson's disease is an autosomal recessive disorder that causes Cu accumulation in the body's liver, brain, and other vital organs (La Fontaine et al.,,2001; Bhattacharya et al., 2005). The study aimed to analyze the clinical variants of WD among males and females (Gao et al., 2019). The data was categorized during the study to calculate the Mean, standard deviation (SD), frequency, and percentage. During the demographic survey of patients, the age was categorized into three intervals ranging from 6 to 20 years. The calculated Mean and standard deviation were 15.6±2.03 as the first interval was from 6 to 10 years, and the calculated frequency percentage was 2 (4.1); the interval ranging from 11 to 15 years had a frequency percentage of 14 (28.6); and the interval ranging from 16 to 20 years was 33 (67.3). Age at disease onset was also categorized into 6–20 years. The calculated Mean and Standard Deviation were 13.5± 2.24. The frequency percentage for ages ranging from 6 to 10 years was 7 (14.3); for 11 to 15, 36 (73.5); and for 16 to 20, it was calculated as 6 (12.2). In the demographic profile, the total females were 20 (40.8) and males were 29 (59.2). The blood group was categorized as A+, A-, B+, B-, O+, and O-. The frequency percentage of obtained A+ individuals was 6 (12.2) for A-3 (6.1), B+ 23 (46.9), B+ 10 (20.4), and O+ 5 (10.2), and for O-2 (4.1), the highest frequency percentage of B+ was observed in the local community that was observed in the demographic survey. The survey was limited to the local population of Pakistan. The participants belonged to different provinces of Pakistan, categorized into Punjabi, Sindhi, KPK, and Balochistan. The frequency percentage of individuals regarding their ethnicity was calculated as Punjabi 21 (42.9), Sindhi 18 (36.7), KPK 1 (2.0), and Balochistan 9 (18.4). The highest percentage of respondents was obtained from Punjabi 21 (42.9%), and the lowest percentage was obtained from KPK 1 (2.0%). The respondents belonged to rural and urban areas, with urban respondents having a frequency percentage calculated at 24 (49.0) and rural respondents possessing a frequency percentage of 25 (51.0), greater than the urban population. By the demographical survey, respondents' education was categorized as middle, matricule, and F.Sc; the majority of respondents were matricule 36 (73.5%), and fewer were from F.Sc. level 3 (6.1%), and the middle educational status of respondents was calculated in frequency percentage 10 (20.4%). Among those where parental cousin marriage exists, it has the lowest frequency percentage, 24 (49.0), compared to those where parental cousin marriage doesn't exist, 25 (51.0). Among those individuals where the disease exists in family history, the calculated frequency percentage is 8 (16.3). Among those where the disease does not exist in family history, the calculated frequency percentage is 41 (83.7). Among those individuals, 24-hour urinary copper was calculated, with a mean and standard deviation of 117.1±191.4. Bilirubin micromol per liter was calculated at a frequency of 3.69±11.96, and for total bilirubin in micromole per litre, the mean and standard deviation were calculated at 1196±6.38. The basic purpose of the research was to statistically analyze the clinical variants of Wilson's disease among males and females to check whether various symptoms appear in males and females.
The purposeful association of gender with various factors was calculated. A total of 49 participants were taken in the study to calculate gender association with PCM and to whom it existed. The respondents had a yes response among those males (8 (27.6) and females (16 (80.0). Among those where parental cousin marriage does not exist, there was a frequency percentage of 25 (51.0), males were 21 (72.4), and females were 4 (20.0). The calculated p-value was 0.000 by applying the chi-square test. It indicates that results are significant at the p-value calculated by Fischer's coefficient by comparing the values to check whether the results are significant or not, or at what percent the results are significant. Higher-frequency populations exist because of frequent first-cousin marriages and a higher mutation frequency. When calculating prevalence from the incidence related to the number of births, estimates were 1:40,000–1:50,000 (Dan et al, 2006; Herrera-Quiñones et al., 2022). In checking the association of gender with family history, the existence of the disease in the family history to which 16.3%were male and 20.7% were female and among those the family history does not exist calculated 83.7%, males were 79.3%, and females were 19.0. The calculated p-value indicated that the results were significant at the 5% level. The yellow sclera symptom existed in 67.3% of the population; among those, 100% were males. The calculated p-value indicated that the results are significant at the 1% level. In three patients, the first manifestation of Wilson's disease was a syndrome in which acute intravascular hemolysis and acute liver failure were associated. This syndrome developed in three periods; the first, lasting 3 to 14 days, was characterized by fatigue and fever (Yarbrough et al., 1947). The symptom of fatigue existed in 37.9% of males and 0.000% of females; it was absent in 2.1% of males and 100.0% of females, indicating that the results are significant at the 1% level. Anoxia appeared in 13.8% of males and 0.00% of females, whereas most of the disease appeared in males compared to females. The p-value was calculated and indicated that the results are significant at the 10% level. The clubbing of the fingers and toes was prominent; the skin showed decided pigmentation of generalized distribution and edema of the lower part of the legs. The liver and spleen were palpable, with constant blood oozing from the gums and intermittent microscopic hematuria (Hansen & Horslen, 2008; Elam et al., 1948). The psychiatric symptoms appeared in most males compared to females; the symptoms were completely absent in the female population, indicating that the result was significant at the 1% level. The symptoms of confusion were 20.7% male and 0.00% female; this symptom was completely absent in females. The 100.0% calculated p-value indicated that the results are significant at the 10% level, so the relationship does not exist. Jaundice is a clinical sign that reflects the accumulation of bilirubin in the blood. It can result from increased bilirubin production, the inability of the liver to conjugate bilirubin, or failure to excrete bilirubin into the biliary tree (Nussinson et al., 2013; Yoshitoshi et al., 2009; Viswanathan et al. 2009), which was present in a greater population of males as compared to females (Kim & Yoo 1998). The calculated p-value indicated that the results are significant at the 1% level, as the p-value was calculated as 0.00. Lower limb edema was present in 10.0% of females and 55.2% of males. The symptom was insignificant, indicating that the absence of such a symptom was recorded in 63.3% of the population (Tu & Blackwell 1967). The results are insignificant, so no relation exists between gender and lower limb edema. The symptoms of Spider nivae are not very common in Wilson's disease, as not much was observed in male and female participants. Abnormal movements were observed in many male participants as compared to females. Among the participants, males were 58.6%, and females were 100.0% (Connor & McDiarmid 2006). The calculated p-value indicated that the results are significant at the 5% level. Chronic liver disease was much more significant among male participants as compared to female participants (Nicastro et al. 2010). The p-value demonstrated that the results are significant at the 5% level. An association of antinuclear antibodies with gender demonstrated that the results are insignificant, and the relation does not exist. The hepatitis B antigen symptom was observed in the majority of females as compared to males (Gower-Rousseau et al., 2009; Czlonkowska et al., 1996). The calculated p-value indicated that the results are insignificant and a relationship does not exist. Fibrosis stage 2-4 was observed in the majority of females as compared to males. calculated p-value demonstrated that the results are significant at the 5% level. The level of Cu was normal in 42.9% of the population, of which females were 90.0%, a much greater frequency percentage than in comparison with males. 10.3% abnormal quantification of copper was observed in 57.1% of the population, of which 10.0% and 89.7% were female and male, respectively (Huster et al., 2012; Gross et al., 1985; Aab et al., 2018).). The p-value showed that the result was significant at the 1% level. Keyser-Fischer rings were observed in 28.6% of individuals among those male participants, with a greater frequency percentage than the female p-value, demonstrating that the relation does not exist. Kayser-Fleischer (kf) rings demonstrate a more significant alteration of Cur metabolism than those who do not have kf rings (Li et al., 2021).
CONCLUSION
The study proved that the factual data from the statistical analysis shows a significant association between clinical variants of Wilson's disease and males and females. It has been investigated that Wilson's disease exists among the local population, and the patient's demographic characteristics have been concluded. This may be due to the excess accumulation of Cu in food consumed by people, which may disturb the function of the liver. However, more significant research related to WD should be undertaken to validate the link between Cu and environmental destruction. It is established that discharged of waste from the industries must be treated before
ACKNOWLEDGMENT
The author appreciated the Department of Bioscience, University of Wah, WahCantt, KPK, for providing research facilities.
REFERENCES
- Aab, A., Abreu, P., Aglietta, M., Albuquerque, I. F. M., Albury, J. M., Allekotte, I., & Huege, T. (2018). Large-scale cosmic-ray anisotropies above 4 EeV measured by the Pierre Auger Observatory. The Astrophysical Journal, 868(1), 4.
- Arredondo, M., & Núñez, M. T. (2005). Iron and copper metabolism. Molecular aspects of medicine, 26(4-5), 313-327.
- Bhattacharya, S., Saha, S. P., Basu, A., & Das, S. K. (2005). A 5 years prospective study of incidence, morbidity and mortality profile of stroke in a rural community of eastern India. Journal of the Indian Medical Association, 103(12), 655-659.
- Birkholz, E. S., & Oetting, T. A. (2009). Kayser-Fleischer Ring: A Systems Based Review of the Ophthalmologist's Role in the Diagnosis of Wilson's Disease.
- Brewer, G. J., & Yuzbasiyan-Gurkan, V. (1992). Wilson disease. Medicine, 71(3), 139-164.
- Chun, S. Y., Reese, T. G., Ouyang, J., Guerin, B., Catana, C., Zhu, X., & El Fakhri, (2012). MRI-based nonrigid motion correction in simultaneous PET/MRI. Journal of Nuclear Medicine, 53(8), 1284-1291.
- Connor, T. H., & McDiarmid, M. A. (2006). Preventing occupational exposures to antineoplastic drugs in health care settings. CA: a cancer journal for clinicians, 56(6), 354-365.
- Czlonkowska, A., Gajda, J., & Rodo, M. (1996). Effects of long-term treatment in Wilson's disease withd-penicillamine and zinc sulphate. Journal of neurology, 243(3), 269-273.
- Dan, Y. Y., Riehle, K. J., Lazaro, C., Teoh, N., Haque, J., Campbell, J. S., & Fausto, (2006). Isolation of multipotent progenitor cells from human fetal liver capable of differentiating into liver and mesenchymal lineages. Proceedings of the National Academy of Sciences, 103(26), 9912-9917.
- Davis, S. Q., & Garrity Jr, E. R. (2007). Organ allocation in lung transplant. Chest, 132(5), 1646-1651.
- de Bie, P., Muller, P., Wijmenga, C., & Klomp, L. W. (2007). Molecular pathogenesis of Wilson and Menkes disease: correlation of mutations with molecular defects and disease phenotypes. Journal of medical genetics, 44(11), 673-688.
- del Rosario, M. A., Davis, M. M., & Chong, S. K. (1998). Wobbly handwriting. The Lancet, 351(9099), 336.
- Eghtesad, B., Nezakatgoo, N., Geraci, L. C., Jabbour, N., Irish, W. D., Marsh, W., & Rakela, J. (1999). Liver transplantation for Wilson's disease: a single‐center experience. Liver Transplantation and Surgery, 5(6), 467-474.
- Elam, J. O., Hemingway, A., Gullickson, G., & Visscher, M. B. (1948). Impairment of pulmonary function in poliomyelitis: oximetric studies in patients with the spinal and bulbar types. Archives of Internal Medicine, 81(5), 649-665.
- Ferenci, P. (2004). diagnosis and current therapy of Wilson's disease. Alimentary pharmacology & therapeutics, 19(2), 157-165.
- Gao, Q., Xu, J., & Bu, X. H. (2019). Recent advances about metal–organic frameworks in the removal of pollutants from wastewater. Coordination Chemistry Reviews, 378, 17-31.
- Gibbs, K., & Walshe, J. M. (1979). A study of the caeruloplasmin concentrations found in 75 patients with Wilson's disease, their kinships and various control groups. QJM: An International Journal of Medicine, 48(3), 447-463.
- Gower-Rousseau, C., Dauchet, L., Vernier-Massouille, G., Tilloy, E., Brazier, F., Merle, V., & Colombel, J. F. (2009). The natural history of pediatric ulcerative colitis: a population-based cohort study. Official Journal of the American College of Gastroenterology| ACG, 104(8), 2008-2088.
- Gross, D. J., Harvey, J. A., Martinec, E., & Rohm, R. (1985). Heterotic string. Physical Review Letters, 54(6), 502.
- Gupta, S. C., Patchva, S., & Aggarwal, B. B. (2013). Therapeutic roles of curcumin: lessons learned from clinical trials. The AAPS journal, 15(1), 195-218.
- Hansen, K., & Horslen, S. (2008). Metabolic liver disease in children. Liver transplantation, 14(4), 391-411.
- Hedera, P. (2017). Update on the clinical management of Wilson's disease. The application of clinical genetics, 10, 9.
- Herrera-Quiñones, G., Da Fieno, A. M., Compta, Y., Forns, X., & Mariño, Z. (2022). Considerations for optimizing Wilson's disease patients' long-term follow-up. Gastroenterología y Hepatología (English Edition).
- Höglinger, G. U., Respondek, G., Stamelou, M., Kurz, C., Josephs, K. A., Lang, A. E., & Bordelon, Y. (2017). Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Movement Disorders, 32(6), 853-864.
- Hung, I. H., Suzuki, M., Yamaguchi, Y., Yuan, D. S., Klausner, R. D., & Gitlin, J. (1997). Biochemical characterization of the Wilson disease protein and functional expression in the yeast Saccharomyces cerevisiae. Journal of Biological Chemistry, 272(34), 21461-21466.
- Huster, D., Kühne, A., Bhattacharjee, A., Raines, L., Jantsch, V., Noe, J., & Lutsenko, S. (2012). Diverse functional properties of Wilson disease ATP7B variants. Gastroenterology, 142(4), 947-956.
- Kim, T. K., & Yoo, E. K. (1998). A Correlational Study on the level of Importance & performance of postpartal Care and its Relationship with Women's Health Status. Korean Journal of Women Health Nursing, 4(2), 145-161.
- Korman, J. D., Volenberg, I., Balko, J., Webster, J., Schiodt, F. V., Squires Jr, R. H., & Pediatric and Adult Acute Liver Failure Study Groups. (2008). Screening for Wilson disease in acute liver failure: a comparison of currently available diagnostic tests. Hepatology, 48(4), 1167-1174.
- La Fontaine, S., Theophilos, M. B., Firth, S. D., Gould, R., Parton, R. G., & Mercer, F. (2001). Effect of the toxic milk mutation (tx) on the function and intracellular localization of Wnd, the murine homologue of the Wilson copper ATPase. Human molecular genetics, 10(4), 361-370.
- Mitra, V., & Metcalf, J. (2009). Metabolic functions of the liver. Anaesthesia & Intensive Care Medicine, 10(7), 334-335.
- Mufti, A. R., Burstein, E., Csomos, R. A., Graf, P. C., Wilkinson, J. C., Dick, R. D., & Duckett, C. S. (2006). XIAP Is a copper binding protein deregulated in Wilson's disease and other copper toxicosis disorders. Molecular cell, 21(6), 775-785.
- Namestnikova, D., Gubskiy, I., Kholodenko, I., Melnikov, P., Sukhinich, K., Gabashvili, A., & Yarygin, K. (2017). Methodological aspects of MRI of transplanted superparamagnetic iron oxide-labeled mesenchymal stem cells in live rat brain. PLoS One, 12(10), e0186717.
- Nicastro, E., Ranucci, G., Vajro, P., Vegnente, A., & Iorio, R. (2010). Re‐ evaluation of the diagnostic criteria for Wilson disease in children with mild liver disease. Hepatology, 52(6), 1948-1956.
- Nussinson, E., Shahbari, A., Shibli, F., Chervinsky, E., Trougouboff, P., & Markel, A. (2013). Diagnostic challenges of Wilson's disease presenting as acute pancreatitis, cholangitis, and jaundice. World journal of hepatology, 5(11), 649.
- Ogra, Y., & Suzuki, K. T. (1998). Targeting of tetrathiomolybdate on the copper accumulating in the liver of LEC rats. Journal of inorganic biochemistry, 70(1), 49-55.
- Parker, G. A., & Picut, C. A. (2005). Liver immunobiology. Toxicologic Pathology, 33(1), 52-62.
- Pasricha, S. R., Black, J., Muthayya, S., Shet, A., Bhat, V., Nagaraj, S., ... & Shet, A. S. (2010). Determinants of anemia among young children in rural India. Pediatrics, 126(1), e140-e149.
- Piel, F. B., Patil, A. P., Howes, R. E., Nyangiri, O. A., Gething, P. W., Dewi, M., & Hay, S. I. (2013). Global epidemiology of sickle haemoglobin in neonates: a contemporary geostatistical model-based map and population estimates. The Lancet, 381(9861), 142-151.
- Plauth, M., Cabre, E., Riggio, O., Assis-Camilo, M., Pirlich, M., Kondrup, J., & Nolte, W. (2006). ESPEN guidelines on enteral nutrition: liver disease. Clinical nutrition, 25(2), 285-294.
- Prashanth, L. K., Sinha, S., Taly, A. B., & Vasudev, M. K. (2010). Do MRI features distinguish Wilson's disease from other early onset extrapyramidal disorders? An analysis of 100 cases. Movement disorders, 25(6), 672-678.
- Roberts, E. A., & Schilsky, M. L. (2008). Diagnosis and treatment of Wilson disease: an update. Hepatology, 47(6), 2089-2111.
- Rudnick, D. A., & Perlmutter, D. H. (2005). Alpha‐1‐antitrypsin deficiency: a new paradigm for hepatocellular carcinoma in genetic liver disease. Hepatology, 42(3), 514-521.
- Schilsky, M. L., Blank, R. R., Czaja, M. J., Zern, M. A., Scheinberg, I. H., Stockert, J., & Sternlieb, I. (1989). Hepatocellular copper toxicity and its attenuation by zinc. The Journal of clinical investigation, 84(5), 1562-1568.
- Svetel, M., Kozić, D., Stefanova, E., Semnic, R., Dragaševič, N., & Kostič, V. S. (2001). Dystonia in Wilson's disease. Movement disorders: official journal of the Movement Disorder Society, 16(4), 719-723.
- Taly, A. B., Meenakshi-Sundaram, S., Sinha, S., Swamy, H. S., & Arunodaya, G. (2007). Wilson disease: description of 282 patients evaluated over 3 decades. Medicine, 86(2), 112-121.
- Tarrier, N., Khan, S., Cater, J., & Picken, A. (2007). The subjective consequences of suffering a first episode psychosis: trauma and suicide behaviour.Social psychiatry and psychiatric epidemiology,42, 29-35.
- Tu, J. B., & Blackwell, R. Q. (1967). Studies on levels of penicillamine-induced cupriuresis in heterozygotes of Wilson's disease. Metabolism, 16(6), 507-513.
- Viswanathan, S. R., Powers, J. T., Einhorn, W., Hoshida, Y., Ng, T. L., Toffanin, S., & Daley, G. Q. (2009). Lin28 promotes transformation and is associated with advanced human malignancies. Nature genetics, 41(7), 843-848.
- Wiebers, D. O., Hollenhorst, R. W., & Goldstein, N. P. (1977, July). The ophthalmologic manifestations of Wilson's disease. In Mayo Clinic Proceedings (Vol. 52, No. 7, pp. 409-416).
- Yarbrough, O. D., Welham, W., Brinton, E. S., & Behnke, A. R. (1947). Symptoms of oxygen poisoning and limits of tolerance at rest and at work. Navy Experimental Diving Unit Panama City Fl.
- Yoshitoshi, E. Y., Takada, Y., Oike, F., Sakamoto, S., Ogawa, K., Kanazawa, H., Uemoto, S. (2009). Long-term outcomes for 32 cases of Wilson's disease after living-donor liver transplantation. Transplantation, 87(2), 261-267.
How to Cite this paper?
APA-7 Style
Arif,
A. (2024). Statistical Analysis of Clinical Variants of Wilson Disease Among Males and Females in Local Population of Wah Cantt, Pakistan. Journal Advances of Nutrition Science and Technology, 4(1-2), 23-34. https://doi.org/10.15228/ANST.2024.v04.i01-2.p03
ACS Style
Arif,
A. Statistical Analysis of Clinical Variants of Wilson Disease Among Males and Females in Local Population of Wah Cantt, Pakistan. J. Adv. Nutri. Sci. Tech. 2024, 4, 23-34. https://doi.org/10.15228/ANST.2024.v04.i01-2.p03
AMA Style
Arif
A. Statistical Analysis of Clinical Variants of Wilson Disease Among Males and Females in Local Population of Wah Cantt, Pakistan. Journal Advances of Nutrition Science and Technology. 2024; 4(1-2): 23-34. https://doi.org/10.15228/ANST.2024.v04.i01-2.p03
Chicago/Turabian Style
Arif, Aleena.
2024. "Statistical Analysis of Clinical Variants of Wilson Disease Among Males and Females in Local Population of Wah Cantt, Pakistan" Journal Advances of Nutrition Science and Technology 4, no. 1-2: 23-34. https://doi.org/10.15228/ANST.2024.v04.i01-2.p03

This work is licensed under a Creative Commons Attribution 4.0 International License.

