Comparative Analysis of Phenolic and Flavonoid Content in Mango Varieties: Evaluating Antioxidant and Antimicrobial Activity
| Received 05 Aug, 2024 |
Accepted 26 Aug, 2024 |
Published 30 Aug, 2024 |
This study reports the antioxidant and antimicrobial attributes of different solvent extracts derived from selected mango varieties. The crude concentrated extracts (CCEs) obtained from Sindhri and Langra exhibited yields ranging from 6.12-23.89 g/100g and 6.64-24.86 g/100g per dry matter, respectively. These CCEs of Sindhri and Langra revealed a significant amount of total phenolic content over the range of 14.43-44.20 GAE (mg/100g) and 14.99-47.57 GAE (mg/100g), respectively. Similarly, the total flavonoid content in CCEs of Sindhri and Langra ranged from 9.85-30.54 CE (mg/100g) and 10.38-36.98 CE (mg/100g), respectively. The recovered CCEs of mango exhibited notable DPPH radical scavenging capacity with IC50 values ranging from 6.78-18.53 µg/mL for Sindhri and 6.11-16.72 µg/mL for Langra. These extracts also exhibited strong ability inhibitory effects (37% to 79%) on the peroxidation process in the linoleic system. Aqueous ethanol (80%) exhibited the highest antimicrobial activity against a variety of pathogenic microorganisms, including Bacillus pumilus, Escherichia coli, Fusarium oxyesporum and Aspergillus niger, while distilled water CCEs displayed the lowest potential. The number of phenolic components detected exceeds those previously reported through HPLC analysis. The findings of this study strongly support the potential utilization of aerial parts of mango as valuable sources of antioxidant and antimicrobial agents.
INTRODUCTION
Free radicals have unpaired electrons, making them highly reactive, unstable and short-lived molecules. As a result, these frequently form bonds with other molecules to achieve stability (Carsono et al., 2022). Free radicals can form when molecules are attacked, leading to breaking chemical bonds and generating these reactive species. Excessive free radical generation and insufficient antioxidant production can lead to oxidative stress (Kashif et al., 2024). Oxidative stress is implicated in numerous diseases, including stroke, cancer, myocardial infarction and diabetes (Anees et al., 2023). Phytochemicals, chemical compounds in plants, possess protective properties (Naz et al., 2023). These phytochemicals are responsible for the antioxidant potential of plants. Antioxidants capture free radicals through chelation, function as scavengers of free radicals and use various mechanisms to prevent lipid oxidation (Kashif et al., 2024). Antioxidants can stop or hinder oxidative damage to molecules. Two main methods of radical deactivation by antioxidant molecules, hydrogen atom transfer and single electron transfer, lead to the same results. The equilibrium between these two mechanisms, which virtually always coexist in all samples, is defined by the antioxidant's pH and structure (Munteanu & Apetrei, 2022).
Plants serve as valuable sources of antioxidants, and herbs have been utilized for medicinal purposes since ancient eras. Each plant has its unique bioactivity (Kashif et al., 2024). Plants are also recognized for their antioxidant properties and benefits to human health (Kruk et al., 2019). Natural sources of antioxidants are preferred due to their affordability and organic nature. In the literature, synthetic antioxidants are noted to be expensive and associated with several adverse effects.
Conversely, conventional extracts from medicinal plants are highly effective and have minimal side effects, making them the preferred choice (). Medicinal plants are abundant worldwide, and there is a wide variety of them in Pakistan. People commonly use them in various forms of herbal medicine to treat many diseases. Pakistan's climate, geographical features, traditional zones and flora are diverse, contributing to the country's rich variety of medicinal plants (Tufail et al., 2020). Medicinal plants have been utilized in healthcare systems since ancient times and have proven effective in treating various ailments (Kashif et al, 2024; Keshav et al., 2019).
The substantial volume, environmental impact and decay of medicinal plant food waste present a significant issue (Jeswani et al., 2021). It is imperative to reduce, reuse and recycle food waste to protect the environment, conserve food resources and promote the sustainability of food system. Many parts of plants, including leaves, fruits and flowers are edible and frequently consumed worldwide (Picot-Allain et al., 2021). Each plant has medicinal potential, which can be explored as a source of molecules of interest to the medicinal industry, even if not every part of every plant has healing properties (Naz et al., 2023). One example of a plant with potential therapeutic benefits is Mangifera indica (Linn.), or mango. Mangifera indica belongs to the Anacardiaceae family and is significant in traditional and Ayurvedic medicine (Nashvia et al., 2022). This fruit is rich in a diverse array of chemical components, including phenolics such as xanthones (predominantly mangiferin), gallates, benzophenones, flavonoids and derivatives of ellagic acid (Asunción-Alvarez et al., 2020).
Mango also contains anthocyanins and carotenoids as phytochemicals. In addition, it is rich in various vitamins, including folic acid, ascorbic acid and niacin. Mango fruits also contain many volatile compounds, such as monoterpenes, esters, lactones and sesquiterpenes, contributing to their sweet and fruity aroma (Kawa-rygielska et al., 2020). Mangoes are frequently consumed fresh but can also be processed to produce juices and pulps, generating substantial waste (Jahurul et al., 2015). Peels are a significant by-product, rarely used in processed food, but can potentially serve as valuable ingredients, as they contain substantial bioactive components. Mango leaves have medicinal applications for treating conditions such as diarrhea, dysentery and colitis (Coelho et al., 2019). Mango bark's aqueous extract has a long history of use in ethnomedicine for addressing various ailments, including diarrhea, diabetes and anemia. It is rich in phenolic substances such as tannins, phenolic acids, benzophenones, xanthones and flavonoids (Vazquez-Olivo et al., 2019).
Medicinal plants contain inherent antioxidants such as phenols, flavonoids and tannins, which are vital in disease prevention. With the growing demand for new natural antioxidants sourced from plants, the abundant presence of these compounds in medicinal plants holds substantial promise for their utilization in pharmaceutical products. Medicinal plants possess valuable properties, including antioxidants, antimicrobials and potential anticancer activities. This study aimed to assess the levels of biologically active compounds and antioxidant capacity across various Mango species in different solvents and concentrations. These solvents encompassed absolute methanol, aqueous methanol, absolute ethanol, aqueous ethanol, chloroform and distilled water extracts derived from various parts of mango varieties (including leaves, bark and peel) through maceration.
MATERIALS AND METHODS
Chemicals and Reagents
Folin-Ciocalteu reagent, aluminum chloride, ammonium thiocyanate, sodium carbonate (anhydrous), linoleic acid, ascorbic acid, gallic acid, ferrous chloride, sodium nitrite, BHT (butylated hydroxytoluene), and DPPH (2, 2-diphenyl-2-picrylhydrazyl) were purchased from Sigma Chemicals. Additional analytical-grade reagents, including ethanol, chloroform and methanol, were obtained from Merck Chemical Company (Darmstadt, Germany). Some chemicals were also purchased from Oxoid Ltd. (Hampshire, United Kingdom) to prepare culture media for antimicrobial tests.
Sample collection and its pretreatment
Different aerial parts (leaves, peel and bark) of selected Mangifera indica (mango) varieties (Sindhri and Langra) were collected from the native district of Multan, South Punjab, Pakistan. A taxonomist from the Department of Botany at the University of Agriculture Faisalabad, Pakistan, assisted in verifying and identifying the mango samples. These samples were chopped into smaller pieces, and finally, the air-dried pieces were stored in plastic bags at -4°C.
Extraction from plant materials
Different parts (leaves, peel and bark) of selected mango varieties (Sindhri and Langra) were washed and rinsed with tap water to remove potential contaminants. The cleaned samples were shade-dried and ground into powder using an electric grinder (mesh 80). 10 g of ground sample was subjected to extraction using 100 mL of various solvents and concentrations, including absolute ethanol, absolute methanol, aqueous ethanol, aqueous methanol, chloroform and distilled water by maceration (16 hours at 26°C).
The recovered green extracts were filtered thrice through Whatman No.1 filter paper to remove any insoluble residue. The resultant extracts were pooled in a new glass vial. Excessive solvents from the crude extract were removed using a rotary evaporator at 45°C, producing concentrated crude extracts (CCEs). These CCEs were then stored at -4°C for further analysis.
Estimation of antioxidant activity of CCEs
The following tests assessed the antioxidant activity of selected parts of different mango varieties.
Estimation of total phenolic content in CCEs
Selected parts of mango were used to determine the total phenolic content using the method described by Yesil-Celiktas et al. (2007). Briefly, CCEs (1.0 mg/mL) were dissolved in 7.5 mL of distilled water, 1.5 mL of 20% sodium carbonate and 0.5 mL of Folin-Ciocalteu reagent. The mixture was then incubated at 40°C for 20 minutes. The absorbance of the solution was measured at 755 nm using a UV-Vis spectrophotometer. The results were expressed as gallic acid equivalents (GAE)/100g of the tested sample.
Estimation of total flavonoid content in CCEs
Selected parts of mango were used to determine the total flavonoid contents following the method reported by Kashif et al. (2024). Briefly, 5.0 mL of distilled water was mixed with CCEs (100 mg/mL) in the presence of 0.3 mL of 5% sodium nitrate. After incubation, the resultant solution was reacted with 2.0 mL of 1.0 M sodium hydroxide and 0.6 mL of 10% aluminum chloride. The absorbance was measured at 510 nm. The concentration of total flavonoid content was determined in catechin equivalents (CE)/100g using a catechin calibration curve.
2, 2-diphenyl-2-picrylhydrazyl radical scavenging analysis
The capacity to neutralize free radicals such as DPPH was assessed using the methodology reported by Tepe et al. (2005). 50 μL sample of each extract with concentration (0.10-5.0 mg/mL) was added to 5.0 mL of a 0.004% DPPH solution in methanol. The resultant solution was incubated for 20 minutes, and the absorbance of the sample and the blank was recorded at 517 nm using Equation 1.
| (1) |
Where As and Ab represent the absorbance of the sample and the blank, respectively.
Inhibition of peroxidation by CCEs
The antioxidant capacity of the recovered CCEs from mango samples was evaluated by assessing their ability to inhibit oxidation of linoleic acid using the methodology given by Azab et al. (2017). For each green extract, 5.0 mg was dissolved in a mixture of 10.0 mL of ethanol (99.8%), 0.13 mL of linoleic acid and 10 mL of sodium phosphate buffer (pH = 7, 0.2 M). Distilled water was added to reach a final volume of 25.0 mL. The degree of oxidation was calculated by measuring the absorbance of the solution after incubation at 40°C (Yen et al., 2000). To the sample solution (0.2 mL), 10.0 mL of 75% ethanol, 0.2 mL of 30% ammonium thiocyanate and 0.2 mL of ferrous chloride solution in 3.5% HCl were sequentially added. The final solution was then incubated at room temperature for 3 minutes while stirring and analyzed using a Spectrophotometer at 500 nm. A control treatment (without plant extract) was also performed, and percentage inhibition was calculated using Equation 2.
| (2) |
Where Ac and As represent the absorbance of the control and sample. This study used positive controls ascorbic acid (C6H8O6) and butylated hydroxytoluene (BHT).
Determination of Alkaloids
10 g of dried powder from each sample was weighed and placed into a 250 mL flask. Subsequently, 100 mL of deionized water was added to the flask. The mixture was shaken for 16 hours and then filtered. For Marme's test, approximately 3 mL of the extracted solution was mixed with several drops of Marme's reagent, which consists of potassium iodide (20 g in 20 mL distilled water) and Cadmium chloride (10 g in 50 mL distilled water). Diluted sulfuric acid was added to the mixture and heated to 40°C for 10 minutes, forming a reddish-brown precipitate.
Antimicrobial activity
Microorganisms tested
The solvent extracts recovered from mango samples were individually tested against a set of pathogenic microorganisms, including Escherichia coli (gram-negative bacteria), Bacillus pumilis (gram-positive bacteria) and fungal strains (Fusarium oxyesporum and Aspergillus niger). The pathogenic microorganism strains were obtained from the Department of Biological Sciences, International Islamic University, Islamabad, Pakistan. Nutrient agarTop of Form Bottom of Form medium and potato dextrose agar (Oxoid, UK) were used to grow the bacterial and fungus strains at 37oC and 30oC, respectively.
Disc diffusion method
The antimicrobial potential of the solvent extracts from the tested samples was measured using disc diffusion, as described by Elfi Susanti & Mulyani (2022). Discs were soaked with each solvent extract (100 mg/mL) and placed on agar plates inoculated with pathogenic microorganisms. Positive controls (amoxicillin and flumequine) and negative controls (without extract) were also processed using the same protocols.
The measurement of the minimum inhibition concentration (MIC) was carried out using the micro-dilution method. MIC represents the exact leaf, bark, and peel extract concentration required to inhibit microorganism growth completely in vitro conditions. Each concentrated solvent extract from the tested samples was diluted in a 5-100 mg/mL range in 96 well plates. A growth control (without plant extract) and sterility control (with plant extract) were also included under similar conditions. A diluted solution of selected plant extracts (20 μL) was added to the culture medium (160 μL) of nutrient broth for bacterial strains and sabouraud dextrose broth for fungal strains. The inoculation of broth culture (20 μL; with 5x105 CFU) of each tested microorganism was performed in the 96-well plate. The plates were incubated at 37oC for 24 hours for bacterial strains and at 30oC for 48 hours for fungal strains. The presence of a white pellet at the bottom of the well indicated microorganism growth. MIC was determined as the dilution where no microorganism growth occurred.
HPLC Analysis
The plant peel was separated using the method described by Öztürk et al. (2007) for sample preparation. Phenolic compounds were analyzed using high-performance liquid chromatography (HPLC). Mango peel (29 g) was mixed with 40 mL of distilled water to produce a juice. The mixture was then centrifuged at 2000g for 15 minutes. After centrifugation, the juice was filtered through a 0.25 mm filter to remove any suspended debris, resulting in a pure juice extract. 15 μL aliquot of the filtered peel sample was injected into an analytical column (Shim-Pak CLC-ODS (C-18), 250 × 4.6 mm; 5 μm particle size), and detection was performed at 280 nm. Phenolic compounds were identified using external standards by comparing them with known standards under identical conditions.
RESULTS AND DISCUSSION
Yield of CCEs
The yield (g/100g) of various green extracts obtained from selected parts of different mango varieties using different solvents and concentrations is shown in Table (1). The yield of plant extracts with antioxidative ingredients depends on the solvent used, with yields ranging from leaves (6.12-12.27 g/100g), peel (15.63-23.89 g/100g) and bark (6.91-16.21 g/100g) per dry matter for the Sindhri variety. For the Langra variety, yields ranged from leaves (6.64-11.89 g/100g), peel (16.51-24.86 g/100g) and bark (7.29-17.86 g/100g) per dry matter. Aqueous ethanol recovered the highest yield, whereas distilled water produced the lowest in both mango varieties. The results of this study indicated that the extract yield varied significantly (p<0.05) depending on the plant component and the solvent used. The highest extract yield was obtained with aqueous ethanol, demonstrating the solvent's superior efficiency in recovering antioxidant constituents (Oktay et al., 2003). A comparison between the two mango varieties revealed that Langra plant samples had a higher yield than Sindhri.
The percentage yield values recovered in this study are lower than those reported in previous research on mangoes (Ling et al., 2009; Sultana et al., 2012). The differences in extract yield may be due to variations in the availability of extractable components, mango variety, fruit maturity and prevailing agroclimatic conditions (Hsu et al., 2006).
| Table 1: | Extract yields from selected parts of the Sindhri and Langra mango plant | |||
Total phenolic and flavonoid content.
Wojdyło et al. (2007) reported that plants with properties such as anti-lipid oxidation potential and anti-carcinogenic effects have gained significant attention in the food industry. Natural antioxidants, particularly phenolics, are commonly derived from plants (Awika et al., 2003). Several studies have shown that total flavonoids and phenolics in fruits and vegetables contribute to their antioxidant activity.
The total flavonoids and phenolics extracted using different solvents from mango are illustrated in Tables (2a & b). The total phenolics (TP) and total flavonoids (TF) extracted from Sindhri mango using various solvents ranged from 14.43-44.20 mg/100g GAE and 9.85-30.54 mg/100g CE. Similarly, TP and TF extracted from Langra mango ranged from 14.99-47.57 mg/100g GAE and 10.38-36.98 mg/100g CE, respectively. Aqueous ethanol extracts showed the highest amounts of TP and TF in both mango varieties. The TP and TF content variation depends on the solvents' effectiveness in extracting antioxidants from the mango plant. The TPC from the plant samples varied significantly (p<0.05) among the solvents tested. Ethanol is often preferred for antioxidant extraction due to its higher efficiency and lower toxicity (Jaffery et al., 2003). This study's total phenolic content values are consistent with previous research on mango plants. However, lower levels of TPC and TFC were noted in certain sections of the mango plant (Sultana et al., 2012).
| Table 2(a): | Total phenolic and total flavonoid content of Sindhri mango plant | |||
| TPC(mg GAE /100g) | TFC(mg CE /100g) | |||||
| Extracting solvents | Leaves | Peel | Bark | Leaves | Peel | Bark |
| Absolute ethanol | 20.22 ± 0.02 | 35.84 ± 0.02 | 18.10 ± 0.01 | 18.77 ± 0.02 | 23.15 ± 0.01 | 11.52 ± 0.01 |
| Aqueous ethanol | 26.87 ± 0.01 | 44.20 ± 0.01 | 22.77 ± 0.01 | 22.97 ± 0.01 | 30.54 ± 0.01 | 16.95 ± 0.02 |
| Absolute methanol | 19.46 ± 0.03 | 34.34 ± 0.01 | 17.83 ± 0.02 | 17.22 ± 0.02 | 21.61 ± 0.02 | 10.70 ± 0.01 |
| Aqueous methanol | 23.13 ± 0.01 | 39.16 ± 0.01 | 20.81 ± 0.01 | 20.03 ± 0.01 | 27.70 ± 0.01 | 14.50 ± 0.02 |
| Distilled water | 19.30 ± 0.02 | 31.31 ± 0.02 | 14.43 ± 0.02 | 16.96 ± 0.02 | 18.13 ± 0.02 | 9.65 ± 0.01 |
| Chloroform | 18.41 ± 0.02 | 33.26 ± 0.02 | 15.98 ± 0.02 | 17.44 ± 0.02 | 19.87 ± 0.01 | 10.03 ± 0.02 |
| Values are the average (mean ± SD) of three replicates, analyzed individually | ||||||
| Table 2(b): | Total phenolic and total flavonoid content of Langra mango plant | |||
DPPH radical scavenging assay:
The stable free radical, DPPH, is known for its deep violet colour, with absorption peaks ranging from 515-528 nm. When it absorbs a proton from hydrogen donors, such as phenolics, it changes colour from violet to yellow. This transition reflects the compound's ability to neutralize free radicals. The DPPH radical scavenging capacity is a widely recognized method to evaluate the antioxidant potential of leaf extracts or similar compounds, and it increases proportionally with higher concentrations of phenolic components or a greater degree of hydroxylation (Sánchez-Moreno et al., 1999).
Mango extracts from selected parts have shown excellent radical scavenging activity, with IC50 values ranging between Sindhri (6.78-18.53 μg/mL) and Langra (6.11-16.72 μg/mL) as detailed in Table (3). Aqueous ethanol extracts exhibited the lowest IC50 value, indicating the most substantial free radical scavenging activity. The results revealed significantly (p < 0.05) higher radical-capturing potential in ethanol extracts than in other solvents. Compared to synthetic antioxidant butylated hydroxytoluene (BTH), the tested mango extracts demonstrated lower scavenging activity. The free radical scavenging values obtained in this study were lower than those reported by Sultana et al. (2012).
| Table 3: | DPPH radical scavenging activity of selected parts of Sindhri and Langra mango plant | |||
| IC50 Value (μg/mL) | ||||||
| Sindhri | Langra | |||||
| Extracting solvents | Leaves | Peel | Bark | Leaves | Peel | Bark |
| Absolute ethanol | 11.28 ± 0.03 | 9.71 ± 0.04 | 12.83 ± 0.04 | 10.02 ± 0.05 | 8.35 ± 0.05 | 11.39 ± 0.04 |
| Aqueous ethanol | 8.34 ± 0.04 | 6.78 ± 0.04 | 9.78 ± 0.02 | 7.52 ± 0.03 | 6.11 ± 0.03 | 8.57 ± 0.02 |
| Absolute methanol | 13.46 ± 0.04 | 10.52 ± 0.04 | 13.66 ± 0.03 | 11.47 ± 0.05 | 9.35 ± 0.03 | 12.22 ± 0.03 |
| Aqueous methanol | 10.53 ± 0.02 | 8.65 ± 0.02 | 11.45 ± 0.05 | 8.75 ± 0.02 | 7.42 ± 0.04 | 9.86 ± 0.05 |
| Distilled water | 17.67 ± 0.05 | 13.56 ± 0.06 | 18.53 ± 0.02 | 15.59 ± 0.04 | 12.73 ± 0.04 | 16.72 ± 0.04 |
| Chloroform | 15.08 ± 0.03 | 11.48 ± 0.04 | 15.76 ± 0.03 | 14.96 ± 0.04 | 11.07 ± 0.02 | 14.39 ± 0.03 |
| Values are the average (mean ± SD) of three replicates, analyzed individually | ||||||
Antioxidant activity in the linoleic acid system
Linoleic acid, an unsaturated fatty acid, undergoes oxidation to produce peroxides. These peroxides subsequently oxidize Fe (II) into Fe (III), forming a complex with thiocyanate anion (SCN-). The concentration of this complex is measured using a UV-vis spectrometer at 500 nm. Higher absorbance values indicate increased peroxide formation, reflecting a lower antioxidant potential, as a greater concentration of peroxides signifies reduced antioxidant activity (Oktay et al., 2003).
The antioxidant potential of selected mango extracts in inhibiting lipid peroxidation is presented in Table (4). The extracts showed varying degrees of inhibition of linoleic acid oxidation, with Sindhri extracts ranging from 37-72% and Langra extracts ranging from 48-79%. Aqueous ethanolic extracts from both mango varieties demonstrated significantly better protection against peroxidation, likely due to their higher concentration of phenolic compounds. Notably, Langra extracts exhibited superior inhibition of linoleic acid oxidation compared to Sindhri extracts, suggesting a higher antioxidant capacity. However, when compared to synthetic antioxidants like BHT, all mango extracts tested showed lower inhibition of linoleic acid oxidation, with significantly less effectiveness (p < 0.05). The oxidation inhibition values obtained in this study were higher than previously reported in the literature (Sultana et al., 2012).
| Table 4: | Antioxidant activity (% inhibition) of Sindhri and Langra mango plant | |||
Antimicrobial activity
Extracts from mango leaf, peel and bark demonstrated antibacterial activity against pathogenic microorganisms, as summarized in Tables (5&6). Bacillus pumilus showed the highest sensitivity among the tested microorganisms, with inhibition zones ranging from 12-14 mm when exposed to extracts from the leaves, peel and bark of the Sindhri mango variety. These results were obtained using the disc diffusion method and determining minimum inhibitory concentration (MIC) values (Table 5a & b). For Bacillus pumilus, the leaf, peel and bark extracts exhibited the lowest MIC values, ranging from 221 to 245 μg/mL, 202 to 237 μg/mL and 222 to 362 μg/mL, respectively (Table 5a). In contrast, Escherichia coli demonstrated lower antimicrobial activity against the leaf, peel and bark extracts of Langra, with comparatively narrower inhibition zones of 2-11 mm, 2-12 mm and 2-7 mm and higher MIC values of 219-281 μg/mL, 198-270 μg/mL and 249-302 μg/mL, respectively (Table 5b). Additionally, bark extracts overtook leaf extracts in antibacterial efficacy against both bacterial and fungal strains.
Regarding the fungus strain Fusarium oxysporum, MIC values ranged from 242-282 μg/mL, 252-308 μg/mL and 259-302 μg/mL for leaf, peel and bark extracts, respectively, with inhibition zones between 2-8 mm, 2-6 mm and 2-7 mm. In comparison, Aspergillus niger exhibited inhibition zones of 2-9 mm, 2-13 mm and 2-10 mm, with MIC values between 237-296 μg/mL, 199-275 μg/mL and 228-289 μg/mL (Table 6a). The variability in the chemical composition of the extracts likely accounts for the differences in antibacterial activity across different components. Previous research has demonstrated that changes in the chemical composition of plant extracts can significantly impact their biological activities. In this study, E. coli was less sensitive to the mango extracts, showing lower inhibitory effects compared to the results documented by Osei-Djarbeng et al. (2020).
| Table 5(a): | Antibacterial activity of extracts of Sindhri mango plant | |||
| Extracting solvents | Bacterial strains | Zone of inhibition (mm) | Minimum inhibitory concentration (μg/mL) | ||||
| Leaves | Peel | Bark | Leaves | Peel | Bark | ||
| Absolute ethanol | Bacillus pumilus | 2 | 3 | 2 | 302 | 277 | 316 |
| Escherichia coli | 8 | 10 | 7 | 224 | 211 | 222 | |
| Aqueous ethanol | Bacillus pumilus | 6 | 5 | 4 | 262 | 252 | 270 |
| Escherichia coli | 12 | 14 | 9 | 221 | 202 | 248 | |
| Absolute methanol | Bacillus pumilus | 2 | 3 | 2 | 318 | 268 | 312 |
| Escherichia coli | 6 | 9 | 5 | 270 | 216 | 275 | |
| Aqueous methanol | Bacillus pumilus | 3 | 4 | 2 | 262 | 249 | 312 |
| Escherichia coli | 8 | 11 | 6 | 302 | 199 | 281 | |
| Distilled water | Bacillus pumilus | 2 | 3 | 1 | 345 | 319 | 362 |
| Escherichia coli | 4 | 8 | 3 | 245 | 237 | 274 | |
| Chloroform | Bacillus pumilus | 3 | 4 | 2 | 269 | 280 | 312 |
| Escherichia coli | 5 | 5 | 3 | 268 | 241 | 299 | |
| Positive control | Bacillus pumilus | _ | 21 | _ | _ | 148 | _ |
| (Amoxicillin) | Escherichia coli | _ | 25 | _ | _ | 98 | _ |
| Values are average (mean ± SD) of three replicates, analyzed individually | |||||||
| Table 5(b): | Antibacterial activity of extracts of Langra mango plan | |||
| Extracting solvents | Bacterial strains | Zone of inhibition (mm) | Minimum inhibitory concentration (μg/mL) | ||||
| Leaves | Peel | Bark | Leaves | Peel | Bark | ||
| Absolute ethanol | Bacillus pumilus | 3 | 4 | 2 | 254 | 240 | 299 |
| Escherichia coli | 9 | 9 | 6 | 220 | 213 | 252 | |
| Aqueous ethanol | Bacillus pumilus | 7 | 7 | 5 | 242 | 235 | 268 |
| Escherichia coli | 11 | 12 | 7 | 219 | 198 | 249 | |
| Absolute methanol | Bacillus pumilus | 2 | 4 | 3 | 319 | 255 | 280 |
| Escherichia coli | 5 | 11 | 4 | 258 | 202 | 262 | |
| Aqueous methanol | Bacillus pumilus | 3 | 6 | 3 | 270 | 249 | 282 |
| Escherichia coli | 8 | 10 | 5 | 238 | 208 | 272 | |
| Distilled water | Bacillus pumilus | 1 | 3 | 2 | 356 | 286 | 305 |
| Escherichia coli | 3 | 7 | 5 | 281 | 233 | 276 | |
| Chloroform | Bacillus pumilus | 2 | 3 | 4 | 302 | 292 | 285 |
| Escherichia coli | 3 | 5 | 2 | 281 | 270 | 302 | |
| Positive control | Bacillus pumilus | _ | 21 | _ | _ | 148 | _ |
| (Amoxicillin) | Escherichia coli | _ | 25 | _ | _ | 98 | _ |
| Values are average (mean ± SD) of three replicates, analyzed individually | |||||||
| Table 6(a): | Antifungal activity of extracts of Sindhri mango plant. | |||
| Extracting solvents | Bacterial strains | Zone of inhibition (mm) | Minimum inhibitory concentration (μg/mL) | ||||
| Leaves | Peel | Bark | Leaves | Peel | Bark | ||
| Absolute ethanol | Aspergillus niger | 8 | 11 | 6 | 250 | 211 | 256 |
| Fusarium oxysporum | 4 | 3 | 2 | 272 | 283 | 277 | |
| Aqueous ethanol | Aspergillus niger | 9 | 13 | 10 | 237 | 199 | 229 |
| Fusarium oxysporum | 3 | 4 | 2 | 282 | 279 | 300 | |
| Absolute methanol | Aspergillus niger | 6 | 7 | 5 | 255 | 261 | 268 |
| Fusarium oxysporum | 3 | 4 | 3 | 279 | 276 | 279 | |
| Aqueous methanol | Aspergillus niger | 7 | 9 | 7 | 260 | 250 | 260 |
| Fusarium oxysporum | 8 | 6 | 7 | 242 | 252 | 259 | |
| Distilled water | Aspergillus niger | 2 | 3 | 2 | 290 | 281 | 275 |
| Fusarium oxysporum | 2 | 3 | 3 | 298 | 298 | 292 | |
| Chloroform | Aspergillus niger | 2 | 3 | 2 | 296 | 275 | 290 |
| Fusarium oxysporum | 3 | 2 | 2 | 284 | 307 | 301 | |
| Values are average (mean ± SD) of three replicates, analyzed individually | |||||||
| Table 6(b): | Antifungal activity of extracts of Langra mango plant | |||
| Extracting solvents | Bacterial strains | Zone of inhibition (mm) | Minimum inhibitory concentration (μg/mL) | ||||
| leaves | fruit | bark | Leaves | peel | bark | ||
| Absolute ethanol | Aspergillus niger | 7 | 14 | 6 | 261 | 193 | 264 |
| Fusarium oxysporum | 6 | 5 | 4 | 261 | 272 | 279 | |
| Aqueous ethanol | Aspergillus niger | 8 | 12 | 10 | 261 | 205 | 240 |
| Fusarium oxysporum | 3 | 7 | 3 | 288 | 259 | 290 | |
| Absolute methanol | Aspergillus niger | 6 | 6 | 7 | 259 | 267 | 252 |
| Fusarium oxysporum | 4 | 7 | 5 | 277 | 255 | 269 | |
| Aqueous methanol | Aspergillus niger | 8 | 8 | 6 | 267 | 264 | 267 |
| Fusarium oxysporum | 9 | 9 | 9 | 247 | 248 | 244 | |
| Distilled water | Aspergillus niger | 4 | 5 | 2 | 278 | 274 | 298 |
| Fusarium oxysporum | 4 | 4 | 3 | 281 | 278 | 289 | |
| Chloroform | Aspergillus niger | 3 | 5 | 3 | 291 | 273 | 294 |
| Fusarium oxysporum | 3 | 4 | 2 | 297 | 277 | 299 | |
| Positive control | Aspergillus niger | 23 | _ | _ | 134 | _ | _ |
| (Amoxicillin) | Fusarium oxysporum | 20 | _ | _ | 160 | _ | _ |
| Values are the average (mean ± SD) of three replicates, analyzed individually | |||||||
Qualitative analysis of alkaloids
The results of Marme's test revealed a distinct variation in alkaloid content among different parts of the Mangifera indica plant. The bark extract demonstrated the highest concentration of alkaloids, significantly more than the leaves. In contrast, no alkaloids were detected in the peel extract (Table 7). The absence suggests that the peel either does not synthesize alkaloids or contains them in quantities below the detection limit of Marme's test. Conversely, the high alkaloid content in the bark indicates its potential as a rich source of these bioactive compounds (Simon et al., 2012).
| Table 7: | Alkaloids of Sindhri and Langra mango plant extracts | |||
| Alkaloids | ||||||
| Solvent Extract | Sindhri | Langra | ||||
| Leaves | Peel | Bark | Leaves | Peel | Bark | |
| Distilled water | + | - | ++ | + | - | ++ |
| + Alkaloids are present, - Alkaloids are absent | ||||||
HPLC analysis
The peels of the tested Mangifera indica plant were analyzed for potent bioactive components, specifically phenolic compounds, using HPLC. Chlorogenic acid was identified as a significant aromatic acid, with a concentration of 112.65 ppm. Other detected aromatic, aromatic acids including quercetin, benzoic, cinnamic, gallic, p-coumaric, caffeic, vanillic and sinapic acids at the concentration of 16.85, 10.17, 6.12, 2.35, 1.71, 1.33, 1.19 and 0.71 ppm, respectively. The promising biological activities, such as antioxidant and antimicrobial potentials, might be attributed to the presence of these phenolic components in M. indica peels, as indicated by the results of correlation analysis. Phytochemicals like phenolic acids are well known for their strong biological properties due to the phenolic moiety (Öztürk et al., 2007).
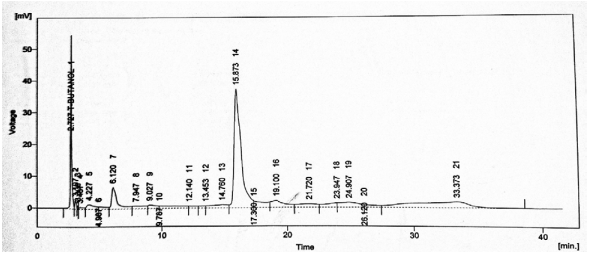
Although the concentration of bioactive components is relatively low, the number of phenolic components detected in this study exceeds those previously reported through HPLC analysis by Safdar et al. (2017). The phytochemical constituents identified in the aqueous extracts via HPLC are presented in Fig. (1), with the corresponding constituents listed in Table (8).
| Table 8: | Phytochemical components and concentration (ppm) in a crude extract of mango peel | |||
| Compound name | Retention Time | Concentration in ppm |
| Quercetin | 2.727 | 16.85 ± 0.04 |
| Gallic acid | 4.227 | 2.35 ± 0.02 |
| Caffeic acid | 12.14 | 1.33 ± 0.02 |
| Vanillic acid | 13.453 | 1.19 ± 0.02 |
| Benzoic acid | 14.76 | 10.17 ± 0.05 |
| Chlorogenic acid | 15.873 | 112.65 ± 0.04 |
| p-coumaric acid | 17.3 | 1.71 ± 0.01 |
| Cinnamic acid | 24.907 | 6.12 ± 0.02 |
| Sinapic acid | 26.12 | 0.71 ± 0.03 |
| Values are average (mean ± SD) of three replicates, analyzed individually | ||
|
Correlation study among TP, TF and biological potential.
The Pearson correlation analysis was conducted at a significance level of P = 0.001 (Tables 9-12). These results revealed a positive correlation between total flavonoids (TF) and total phenolics (TP) with both inhibition potential and antimicrobial properties in the Sindhri and Langra mango varieties. The correlation coefficient for inhibition potential and antimicrobial properties were P = 0.917 and 0.233-0.863, P = 0.953 and 0.144-0.897, P = 0.965 and 0.643-0.923, P = 0.998 and 0.592-0.837, respectively. Conversely, the IC50 values were negatively correlated with TPC and TFC values, with correlation coefficients of P=-0.937, P= -0.913, P=-0.876 and P= -0.811, respectively.
The aerial parts of mango plants from Multan, Pakistan, were investigated for their nutritional content and potential bioactive compounds, driven by increasing interest in their pharmacological benefits. This study represents the first characterization of these selected parts, confirming the presence of phenolic antioxidant compounds. These findings significantly contribute to scientific knowledge by providing evidence of phenolic antioxidants in these parts, showing notable biological activities that were previously underexplored. The results of this study support the potential use of mango aerial parts in various nutraceutical formulations and functional foods, offering multiple health benefits.
| Table 9: | Correlation analysis between biological activities and TP of Sindhri peel | |||
| Table 10: | Correlation analysis between biological activities and TF of Sindhri peel | |||
| Table 11: | Correlation analysis between biological activities and TP of Langra peel | |||
| Table 12: | Correlation analysis between biological activities and TF of Langra peel | |||
CONCLUSION
In this study, the extraction from selected mango varieties using different solvents yielded various chemical compounds, resulting in distinct antioxidant and antimicrobial properties. These variations are influenced by the solvent type and the specific mango part used, which affect the extraction of particular bioactive compounds. We concluded that selecting the appropriate extraction technique is crucial for maximizing the yield of potent antioxidant compounds from mango plant material. We strongly recommend a comprehensive follow-up investigation focused on these bioactive compounds' structural elucidation, isolation and in vivo biological activities in selected mango parts.
ACKNOWLEDGEMENT
Authors are thankful to the Department of Chemistry, International Islamic University, Islamabad; Department of Chemistry, Division of Science and Technology, University of Education, Lahore; Department of Chemistry, Government College University, Faisalabad and Department of Chemistry, Abdul Wali Khan University, Mardan, Pakistan, for providing the research facilities to conduct this project.
CONFLICT OF INTEREST
It is declared that there is no conflict of interest among authors.
REFERENCES
- Anees Ali Jafri, S., Mehmood Khalid, Z., Rizwan Khan, M., Ashraf, S., Ahmad, N., Mahmoud Karami, A., Rafique, E., Ouladsmane, M., Mohammad Saad Al Suliman, N., & Aslam, S. (2023). Evaluation of some essential traditional medicinal plants for their potential free scavenging and antioxidant properties. Journal of King Saud University - Science, 35(3).
- Asunción-Alvarez, R. O. Y.-J. D., M. Quispe-Díaz, I., Palacios, J., Bórquez, J., Simirgiotis, M. J., Perveen, S., Nwokocha, C. R., Cifuentes, F., & Paredes, A. (2020). Metabolomic Profiling of Mango (Mangifera indica Linn) Leaf Extract and Its Intestinal Protective Effect and Antioxidant Activity in Different Biological Models. Molecules, 25, 1–19.
- Awika, J. M., Rooney, L. W., Wu, X., Prior, R. L., & Cisneros-Zevallos, L. (2003). Screening Methods to Measure Antioxidant Activity of Sorghum (Sorghum bicolor) and Sorghum Products. Journal of Agricultural and Food Chemistry, 51(23), 6657–6662.
- Azab, A. E., Albasha, M. O., & Elsayed, A. S. I. (2017). Prevention of Nephropathy by Some Natural Sources of Antioxidants. Yangtze Medicine, 01(04), 235–266.
- Carsono, N., Tumilaar, S. G., Kurnia, D., Latipudin, D., & Satari, M. H. (2022). A Review of Bioactive Compounds and Antioxidant Activity Properties of Piper Species. Molecules, 27(19), 1–22.
- Coelho, E. M., De Souza, M. E. A. O., Corrêa, L. C., Viana, A. C., Azevêdo, L. C. De, & Lima, M. D. S. (2019). Bioactive compounds and antioxidant activity of mango peel liqueurs (Mangifera indica L.) produced by different methods of maceration. Antioxidants, 8(4), 1–11.
- Elfi Susanti, V. H., & Mulyani, S. (2022). Green Synthesis, Characterization, and Antibacterial Activity of Methoxy Chalcones. Rasayan Journal of Chemistry, 15(4), 2459–2465.
- FO, A., lBO, D., & TA, I. (2022). Phytochemical Analysis and Antioxidant Activities of Hot and Cold Water Extracts of Mango (Mangifera indica L.) Leaf and Stem Bark. University of Lagos Journal of Basic Medical Sciences, 1(2).
- Gautam, V. S., Singh, A., Kumari, P., Nishad, J. H., Kumar, J., Yadav, M., Bharti, R., Prajapati, P., & Kharwar, R. N. (2022). Phenolic and flavonoid contents and antioxidant activity of an endophytic fungus Nigrospora sphaerica (EHL2), inhabiting the medicinal plant Euphorbia hirta (dudhi) L. Archives of Microbiology, 204(2), 1–13.
- Ghayas, S., Hannan, A., & Rizwani, G. H. (2022). Phytochemical, Antioxidant, Toxicological, and Pharmaceutical Evaluation of Polyherbal Formulation: Irochel. Dose-Response, 20(1), 1–17.
- Hassan, A., Khan Mohmand, N. Z., Ullah, H., & Hussain, A. (2022). Antioxidant, Antidiabetic, and Antihypertension Inhibitory Potentials of Phenolic Rich Medicinal Plants. Journal of Chemistry, 2022.
- Hsu, B., Coupar, I. M., & Ng, K. (2006). Antioxidant activity of hot water extract from the fruit of the Doum palm, Hyphaene thebaica. Food Chemistry, 98(2), 317–328.
- Jaffery, E. H., Brown, A. F., Kurilich, A. C., Keck, A. S., Matusheski, N., Klein, B. P., & Juvik, J. A. (2003). Variation in content of bioactive components in broccoli. Journal of Food Composition and Analysis, 16(3), 323–330.
- Jahurul, M. H. A., Zaidul, I. S. M., Ghafoor, K., Al-Juhaimi, F. Y., Nyam, K. L., Norulaini, N. A. N., Sahena, F., & Mohd Omar, A. K. (2015). Mango (Mangifera indica L.) by-products and their valuable components: A review. Food Chemistry, 183, 173–180.
- Jeswani, H. K., Figueroa-Torres, G., & Azapagic, A. (2021). The extent of food waste generation in the UK and its environmental impacts. Sustainable Production and Consumption, 26, 532–547.
- Kashif, A. R., Naz, S., Usman, A., Javed, N., Younas, M. U., & Zahoor, A. (2024). In vitro study of antioxidant and antimicrobial potential of Moringa oleifera leaves as a green food preservative in chicken burger Journal Advances of Nutrition Science & Technology (ANST),4. .
- Kawa-rygielska, J., Szumny, A., Czubaszek, A., & Gasi, A. (2020). with Addition of Mango Fruit ( Mangifera Indica ).
- Keshav, A., Mazumdar, B., & Sharma, A. (2019). Phytochemical analysis and antioxidant activity of Colocasia esculenta (L.) leaves. International Journal of Chemical and Molecular Engineering, 13(1), 20–23.
- Kruk, J., Aboul-Enein, H. Y., Kładna, A., & Bowser, J. E. (2019). Oxidative stress in biological systems and its relation with pathophysiological functions: the effect of physical activity on cellular redox homeostasis. Free Radical Research, 53(5), 497–521.
- Ling, L. T., Yap, S. A., Radhakrishnan, A. K., Subramaniam, T., Cheng, H. M., & Palanisamy, U. D. (2009). Standardised Mangifera indica extract is an ideal antioxidant. Food Chemistry, 113(4), 1154–1159.
- Simon, M. K., Nafanda, W. D., & Obeta, S. S. (2012). In-vivo evaluation for anthelmintic effect of alkaloids extracted from the stem bark of Afzelia africana in rats.Journal of Advanced Scientific Research,3(01), 100-104.
- Munir, N., Mahmood, Z., Shahid, M., Afzal, M. N., Jahangir, M., Ali Shah, S. M., Tahir, I. M., Riaz, M., Hussain, S., Akram, M., & Yousaf, F. (2022). Withania somnifera Chemical Constituents' In Vitro Antioxidant Potential and Their Response on Spermatozoa Parameters. Dose-Response, 20(1).
- Munteanu, I. G., & Apetrei, C. (2022). A Review on Electrochemical Sensors and Biosensors Used in Assessing Antioxidant Activity. Antioxidants, 11(3).
- Nashvia Adin, S., Gupta, I., Aqil, M., Mujeeb, M., & Ahad, A. (2022). BBD Driven Optimization of Extraction of Therapeutically Active Xanthanoid Mangiferin from Mangifera indica L. Leaves and its Antioxidant Activity. Pharmacognosy Research, 15(1), 84–93.
- Naz, S., Kashif, A. R., Tawab, A., Rasool, M. Z., Rauf, A., Hussain, S., & Khan, U. (2023). Assessment of the Antidiabetic Properties of Essential Oil from Cannabis sativa. Journal Advances of Nutrition Science and Technology, 3(3–4), 78–86.
- Naz, S., Rasheed, M. N., Saeed, J., . A., Ghuffar, A., Kashif, A. R., & Waheed, R. (2023). Isolation of Bioactive compounds from the essential oil of Jambolan (Syzygium cumini) and Invitro evaluation of biological potential. Journal Advances of Nutrition Science and Technology, 3(1–2), 15–23.
- Ng, Z. X., Yong, P. H., & Lim, S. Y. (2020). Customized drying treatments increased the extraction of phytochemicals and antioxidant activity from economically viable medicinal plants. Industrial Crops and Products, 155(April), 112815.
- Oktay, M., Gülçin, I., & Küfrevioǧlu, Ö. I. (2003). Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts. Lwt, 36(2), 263–271.
- Osei-Djarbeng, S. N., Kwarteng, R. O., Osei-Asante, S., & Owusu-Dapaah, G. (2020). Comparative antimicrobial activities of ethanolic extracts of leaves, seed and stem bark of Mangifera indica (Mango). ~ 1240 ~ Journal of Pharmacognosy and Phytochemistry, 9(1), 1240–1243.
- Öztürk, N., Tunçel, M., & Tunçel, N. B. (2007). Determination of phenolic acids by a modified HPLC: Its application to various plant materials. Journal of Liquid Chromatography and Related Technologies, 30(4), 587–596.
- Picot-Allain, C., Mahomoodally, M. F., Ak, G., & Zengin, G. (2021). Conventional versus green extraction techniques — a comparative perspective. Current Opinion in Food Science, 40, 144–156.
- Safdar, M. N., Kausar, T., & Nadeem, M. (2017). Comparison of Ultrasound and Maceration Techniques for the Extraction of Polyphenols from the Mango Peel. Journal of Food Processing and Preservation, 41(4).
- Sánchez-Moreno, C., A. Larrauri, J., & Saura-Calixto, F. (1999). Free radical scavenging capacity and inhibition of lipid oxidation of wines, grape juices and related polyphenolic constituents. Food Research International, 32(6), 407–412.
- Sultana, B., Hussain, Z., Asif, M., & Munir, A. (2012). Investigation on the Antioxidant Activity of Leaves, Peels, Stems Bark, and Kernel of Mango (Mangifera indica L.). Journal of Food Science, 77(8), 849–852.
- Tepe, B., Daferera, D., Sokmen, A., Sokmen, M., & Polissiou, M. (2005). Antimicrobial and antioxidant activities of the essential oil and various extracts of Salvia tomentosa Miller (Lamiaceae). Food Chemistry, 90(3), 333–340.
- Tufail, M., Hussain, K., Nawaz, K., Bhatti, K. H., Yasin, G., & Ali, S. S. (2020). Ethnobotanical survey of important wild medicinal plants of tehsil Gojra, district toba tek singh, Punjab, Pakistan. Ethnobotany Research and Applications, 20, 1–14.
- Vazquez-Olivo, G., Antunes-Ricardo, M., Gutiérrez-Uribe, J. A., Osuna-Enciso, T., León-Félix, J., & Heredia, J. B. (2019). Cellular antioxidant activity and in vitro intestinal permeability of phenolic compounds from four varieties of mango bark (Mangifera indica L.). Journal of the Science of Food and Agriculture, 99(7), 3481–3489.
- Wetchakul, P., Chonsut, P., Punsawad, C., & Sanpinit, S. (2022). LC-QTOF-MS Characterization, Antioxidant Activity, and In Vitro Toxicity of Medicinal Plants from the Tri-Than-Thip Remedy. Evidence-Based Complementary and Alternative Medicine, 2022.
- Wojdyło, A., Oszmiański, J., & Czemerys, R. (2007). Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chemistry, 105(3), 940–949.
- Yen, G. C., Duh, P. Der, & Chuang, D. Y. (2000). Antioxidant activity of anthraquinones and anthrone. Food Chemistry, 70(4), 437–441.
- Yesil-Celiktas, O., Nartop, P., Gurel, A., Bedir, E., & Vardar-Sukan, F. (2007). Determination of phenolic content and antioxidant activity of extracts obtained from Rosmarinus officinalis' calli. Journal of Plant Physiology, 164(11), 1536–1542.
How to Cite this paper?
APA-7 Style
Kashif,
A.R., Batool,
K., Rabbani,
S.M., Javed,
N., Asadullah,
M., Yaseen,
S., Bibi,
H. (2024). Comparative Analysis of Phenolic and Flavonoid Content in Mango Varieties: Evaluating Antioxidant and Antimicrobial Activity. Journal Advances of Nutrition Science and Technology, 4(3-4), 52-65. https://doi.org/10.15228/ANST.2024.v04.i03-4.p07
ACS Style
Kashif,
A.R.; Batool,
K.; Rabbani,
S.M.; Javed,
N.; Asadullah,
M.; Yaseen,
S.; Bibi,
H. Comparative Analysis of Phenolic and Flavonoid Content in Mango Varieties: Evaluating Antioxidant and Antimicrobial Activity. J. Adv. Nutri. Sci. Tech. 2024, 4, 52-65. https://doi.org/10.15228/ANST.2024.v04.i03-4.p07
AMA Style
Kashif
AR, Batool
K, Rabbani
SM, Javed
N, Asadullah
M, Yaseen
S, Bibi
H. Comparative Analysis of Phenolic and Flavonoid Content in Mango Varieties: Evaluating Antioxidant and Antimicrobial Activity. Journal Advances of Nutrition Science and Technology. 2024; 4(3-4): 52-65. https://doi.org/10.15228/ANST.2024.v04.i03-4.p07
Chicago/Turabian Style
Kashif, Ali, Raza, Kinza Batool, Sheza Marsa Rabbani, Nighat Javed, Muhammad Asadullah, Sohail Yaseen, and Haleema Bibi.
2024. "Comparative Analysis of Phenolic and Flavonoid Content in Mango Varieties: Evaluating Antioxidant and Antimicrobial Activity" Journal Advances of Nutrition Science and Technology 4, no. 3-4: 52-65. https://doi.org/10.15228/ANST.2024.v04.i03-4.p07

This work is licensed under a Creative Commons Attribution 4.0 International License.


