Rhizobacteria Containing ACC Deaminase Enhance Plant Growth and Tolerance Under Abiotic Stress Conditions
| Received 25 Nov, 2023 |
Accepted 27 Dec, 2023 |
Published 31 Dec, 2023 |
Various abiotic stresses affect plants in the environment, and the impact may be impaired by global climate change soon. Plants produce ethylene in response to almost all environmental stresses, which is detrimental to the viability of the plant. Thus, controlling the production of stress ethylene in plants is emerging as an intriguing approach to boost crop production and mitigate the unwanted effects of abiotic stressors. Plants use 1-Aminocyclopropane-1-carboxylic acid (ACC) as a precursor to produce ethylene. It is well known that rhizobacteria that promote plant growth and have ACC deaminase activity can regulate plant growth in extreme conditions by lowering the levels of stress ethylene in plants. Researchers suggest that rhizobacteria that produce ACC deaminase can help plant development under varying environmental conditions, and bacteria with ACC deaminase could play a pivotal role in conferring tolerance and adaptation in plants to various abiotic stressors, indicating their great potential in addition to microbial plant biostimulants. In the present study, we aimed to describe the mechanism of ACC deaminase-producing rhizobacteria in plant growth promotion and tolerance to abiotic stress.
INTRODUCTION
Many abiotic stresses, including drought, salinity, flooding, heat and heavy metals, negatively impact crop plants growth and productivity (Ali & Kim, 2018; Ali et al., 2022; Qureshi et al., 2022). These stresses cause more than 70% yield losses in important food crops (Mantri et al., 2012; Naing et al., 2021). In the near future, the available cultivable land area may shrink because of global climate change, which increases the amount of uncultivable land area. In addition, it is predicted that by 2070, there will be 10 billion people on the earth's planet, endangering food security worldwide and necessitating the promotion of enhanced crop productivity to meet the challenge (Goswami et al., 2016). Hence, it is imperative to mitigate the negative impacts of environmental stressors, increase agricultural productivity, and provide sustainable food for the world's population.
It is well-known that higher amount of ethylene, also known as stress ethylene, which has a deleterious impact on plant physiology, is produced when plants are under abiotic stress, which also significantly affects the physiological and biochemical functions of plants (Glick, 2014; Khan et al., 2019; Qureshi & Ahmed, 2022). The application of PGPR, which significantly aids in the growth and development of plants, is one of the practical strategies for reducing the negative impacts of environmental stresses and improving crop yield under adverse environmental conditions (Ali, et al., 2018; Chieb & Gachomo, 2023; Waheed et al., 2023). Under environmental stress conditions, rhizobacteria that produce ACC deaminase have a clear advantage over PGPRs lacking this enzyme because they are considerably better at enhancing plants' physiological parameters and protecting them from the detrimental effects of higher ethylene concentrations. Rhizospheric bacteria that carry ACC deaminase can be employed as a prolific inoculum to improve the growth and productivity ofcropplants under adverse conditions. This approach can be applied to sustainable agriculture.
The growth of plants can be enhanced by PGPR directly or indirectly (Ali et al., 2022). One way that bacteria indirectly promote plant growth is by preventing phytopathogens from inhibiting the growth and development of plants. On the other hand, direct stimulation may involve providing plants with soluble phosphate and phytohormones and fixing atmospheric nitrogen and iron that bacterial siderophores have sequestered. Additionally, many PGPRs can make plants more resistant to biotic and abiotic stressors. ACC deaminase-producing bacteria have been shown in some studies to play a symbiotic function in both abiotic stress tolerance and plant growth promotion in the native rhizosphere (Murali et al., 2021). More recently, Moon and Ali (2022b) reported S. fonticola (S1T1) as a multi-trait PGPR with ACC deaminase, Indole-3-Acetic Acid (IAA), siderophore, and phosphate solubilization activities (Moon & Ali, 2022b). The application of ACC deaminase-producing bacteria (PGPR) as biofertilizers to enhance plant development has been well-studiedrecently. It has been proposed that foliar application of PGPR can also boost the content of macro- and micronutrients, develop systemic resistance to infections, and endure environmental stressors.
In the present article, we aim to discuss the synthesis of ACC in plants, the role of ACC deaminase-producing rhizobacteria, their mechanism in plant growth promotion, and abiotic stress tolerance.
Rhizospheric bacteria
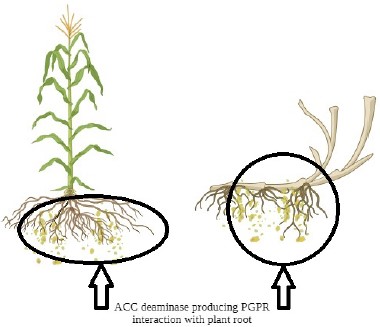
A collection of rhizosphere bacteria capable of colonizing the root environment is represented by the term "rhizobacteria," which refers to the limited zone of soil immediately around the root system (Walker et al., 2003). Plant roots generate, accumulate, and exude a wide range of substances, providing support and enabling the absorption of available nutrients and water in the rhizospheric region and interacting with rhizobacteria (Figure 1). These substances released by plant roots serve as chemical attractants for microbial communities in the soil. Generally, the substances released into the soil by roots are referred to as root exudates. Rhizobacteria that live in the rhizosphere mitigate the effects of abiotic stresses on plants by means of several different mechanisms, such as changes in phytohormone levels, ACC deaminase production, modifications to metabolism, defences against antioxidants, exopolysaccharides produced by the bacteria, and protection and enhancement of root growth. Under stress, these microbes can enhance plant metabolites' photosynthetic, carbohydrate, and protein content and modify their expression, increasing yield-related parameters.
|
The physiological state, type of plant, and microbe determine these exudates' chemical composition. Additionally, rhizosphere microbial activity modifies the quantity and quality of root exudates by influencing plant nutrition availability and rooting patterns. Microorganisms in the area digest some of these plant-derived tiny organic molecules as carbon and nitrogen sources. Plants for growth and development then reabsorb particular molecules that are targeted toward microbes (Kang et al., 2010). The rhizosphere consists of three distinct yet interdependent elements: the rhizosphere, the rhizoplane, and the root itself. The zone of soil affected by roots through the release of substrates that impact microbial activity is known as the rhizosphere. However, because many microorganisms also inhabit the root tissues, the root is a system component. On the other hand, the rhizoplane is the root's surface that includes the soil particles that adhere firmly to the root (Moon & Ali, 2022a). When a root affects the surrounding soil volume, this is known as rhizosphere colonization.
On the other hand, root colonization refers to the microbial colonization of the rhizoplane and root tissues. PGPRs improve the uptake of water and nutrients from the soil, which leads to the growth of plants, even in adverse conditions. Some recent studies revealed that ACC deaminase producing rhizobacteria mitigate the water stress impacts on different plants, including wheat. Moreover, these bacteria can mobilize nutrients by producing other enzymes such as phosphatases, organic acids and siderophores. They also reduce the accumulation of Na+ during salt stress, enhancing phosphate activities and nitrate reductase under water-stressed conditions. Additionally, by producing antagonistic chemicals, they decrease the harmful effects of pathogenic organisms, indirectly promoting plant growth.
PGPR Mechanism in Plant Growth Promotion
A diverse set of bacteria known as PGPR attaches to plantroots and promotes plant growth in a variety of ways. Complex interactions between rhizobacteria have been shown to affect plant productivity and health. The use of these microbes to enhance plant growth has been thoroughly studied recently. Usually, PGPR action strategies have been divided into direct and indirect processes. Indirect processes are typically less evident than direct mechanisms, yet they nevertheless have a difference. The ones occur within the plant and immediately impact its metabolism, whereas direct mechanisms occur outside the plant (Naing et al., 2021). The plant's defence metabolic pathways must participate in the last step because they transduce the signal that the bacteria affect the plant supply. Thus, the term "direct mechanisms" refers to processes that affect the balance of growth regulators released by plants, either directly from the microorganisms or indirectly through their function as a sink for the hormones the plants release, as well as processes that boost the plant's capacity for adaptation through changes in metabolism (Qureshi & Ahmed, 2022). This group includes two significant mechanisms: protection against high saline environments and generation of systemic resistance to plant diseases. This description lists indirect mechanisms that increase the plant's access to nutrients, the prevention of microorganisms that damage the plant, and enhanced nitrogen availability due to free nitrogen fixation in the rhizosphere. More importantly, PGPR uses a greater number of indirect processes such as ACC deaminase production, phosphate solubilization, siderophore production, compounds associated to infections are hydrolyzed, synthesis of enzymes that can break down fungal cell walls.
The primary method of achieving phosphate solubilization by bacterial action is soil acidification through bacteria release of organic acid. These rhizobacteria can chelate the phosphate cations to produce organic acids that plant roots can use. PGPR can also help in the acquisition of iron by generating siderophores, which are organic molecules with a lower molecular weight when iron is deficient. PGPR-producing siderophores lower metal ion availability and prevent harmful metal accumulation in the soil; they are essential for plant survival (Mellidou & Karamanoli, 2022).
PGPR Mechanism for Stress Tolerance
Various PGPRs have been identified to have the ability to lower the level of nitrogen supplementation needed for plant growth. This can be accomplished directly by mobilizing and fixing nitrogen (N) or indirectly by supporting the N-fixers through their secretions (Mellidou & Karamanoli, 2022). Moreover,99% of naturally occurring P is inorganic. Inorganic P that solubilizes PGPR represents another potential for biofertilizer research. P is primarily soluble in soil due to bacterial emission of organic acid, which acidifies the soil. By preventing infections from getting iron, siderophores can also assist plants in preventing the onset of illness. A variety of bacterial species can produce siderophores. Because they lower the availability of metal ions and shield plants from the harmful buildup of metals in the soil, PGPR-producing siderophores are essential for plant survival when exposed to metal stress.
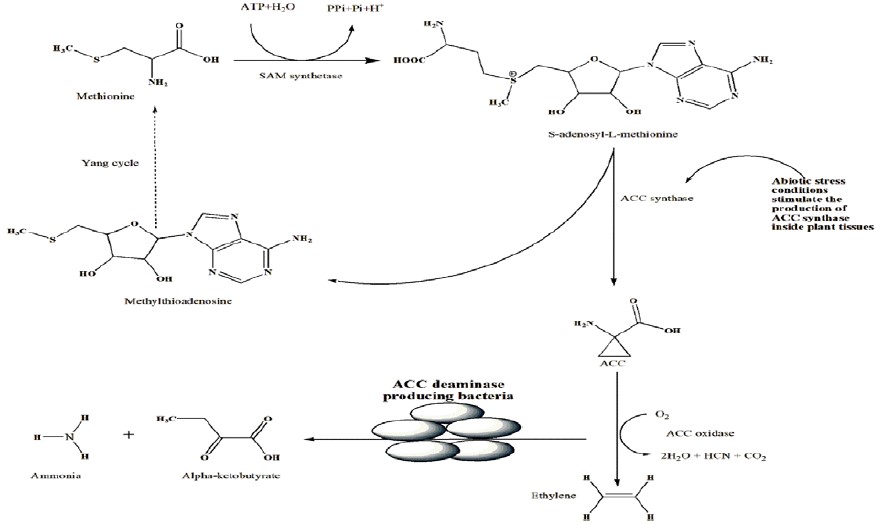
Moreover, PGP strains increase the absorption of metals, including zinc. Bacterial ACC deaminase has a creative function that lowers the amount of ACC secreted and hydrolyzes it into ammonia and α-ketobutyrate to mitigate the adverse effects of stress ethylene on the host plant (Figure 2) (Glick, 2014; Moon & Ali, 2022a; Santoyo et al., 2021). Figure 2 adopted the basic concept from previously published articles (Ali & Kim, 2018; Moon & Ali, 2022a; Moon & Ali, 2022). The ACC deaminase enzyme can scavenge plant ACC, which is present in PGPR. Certain strains of Bacillus amyloliquefaciens, Burkholderia phytofirmans, and Pseudomonas oryzihabitans are among the PGPR that can induce root elongation in their host plants because they contain the enzyme. It has been observed that ACC-deaminase-containing inoculation improves plant development in rice, wheat, beans, and tomatoes when exposed to salt stress or drought stress (Mellidou & Karamanoli, 2022).
There are two ways that PGPR may affect the growth and development of plants: directly or indirectly. By reducing or eliminating some of the harmful effects of a phytopathogenic organism through one or more methods, bacteria indirectly promote the growth of plants. Conversely, PGPR typically involves giving the plant a substance that the bacterium produces or making it easier to absorb nutrients from the surrounding environment to directly promote plant growth and development (Chieb & Gachomo, 2023). The application of these rhizobacteria can be helpful in replacing the application of various chemicals on agricultural lands (Glick, 2014).
|
Plant tissues contain a moderate amount of shootapical meristem (SAM), is converted into ACC and MTA by the enzyme ACC synthase tosynthesize ethylene. The amino acid methionine gets generated when the MTA produced as a byproduct of this reaction is recycled (Figure 2). As a result, even at higher rates of ethylene synthesis, the amount of methionine in a plant cell can stay relatively constant. According to some scientists, the placement, or rate-limiting, step in ethylene biosynthesis is the synthesis of ACC from SAM. Since the genes encoding this enzyme belong to a multigene family, many virtually identical ACC synthase enzymes are present in a plant cell. The bacteria with ACC deaminase convert stress ethylene into ammonia and alpha-ketobutyrate and alleviate the stressful conditions on plants. There have been severalreadily available biocontrol PGPR strains. There are some other characteristics of microbes associated with plants. On the other hand, some pathogenic flora cause disease conditions, while some contribute to alleviating stressful conditions.
The following characteristics are linked to the biocontrol of microbes associated with plants: (i) synthesis of antibiotics (ii) secretion of siderophores that bind iron, (iii) synthesis of low molecular weight metabolites, (iv) synthesis of enzymes, such as lipase, protease, or chitinase (v) surpassing phytopathogens in the competition for resources and root surface niches (vi) utilizing the enzyme ACC deaminase to reduce the amount of stress ethylene produced in plants. Various PGPRs can directly supportthe growth of their plant hosts in severalways, including(i) fixing atmospheric nitrogen, (ii) synthesizing siderophores, (iii) synthesizing different phytohormones, (iv) providing mechanisms for the solubilization of minerals like phosphorus, and (v) synthesizing enzymes that can regulate plant growth and development (Penrose & Glick, 2003). A specific bacterium may use one or more of these strategies to assist in the growth and development of plants. Furthermore, a bacterium may use distinct characteristics during the plant's life cycle stages. For instance, a PGPR may reduce the plant's ethylene levels after seed germination, which would lessen the ethylene inhibition of seedling root length (Gupta & Pandey, 2019). Bacteria expressing ACC deaminase can protect plants against several stressors (Nascimento et al., 2012).More recently, from the rhizosphere soil of cluster beans (Cyamopsis tetragonoloba), Jain and Saraf (2023) isolated and discovered drought-tolerant ACC deaminase-producing PGPR. They also studied the effects of bacterial isolates on plant growth, nodulation, and soil characteristics of cluster beans under drought.
Plants exposed to various biotic and abiotic stressors become more resilient to the stressful conditions upon inoculating ACC deaminase-producing bacteria. Rhizobacteria that promote plant growth and have ACC deaminase activity are known to regulate plant growth in extreme conditions by reducing ethylene concentrations in plants; for this reason, they are sometimes referred to as "stress modulators." In various legumes surviving abiotic stress, ethylene is also known to decrease the formation of nodules. The study of Cedeño-García et al., (2018) revealed bacterial strains that promote plant growth were isolated from the alfalfa rhizosphere, and the strains demonstrated growth-promoting characteristics, including the production of indole acetic acid, the solubilization of phosphate, and the activity of ACC deaminase. Under greenhouse conditions, alfalfa plants display dramatically improved early nodulation due to the rhizosphere-associated bacteria's ACC deaminase activity.
Similarly, Singh et al., (2023) reported the role of ACC deaminase-producing PGPR in mitigating salt stress in Ocimum sanctum. More recently, Shahid et al., (2023) reported that ACC deaminase-producing bacterial strains are preferable to other common bacterial strains because they can grow in large enough quantities in new and challenging conditions. Strong PGPR strains improve crop yield and growth in abiotic stressconditions. A significant paradigm shift in agricultural operations is desperately needed, given the numerous severe environmental concerns arising fromnatural and artificial sources when using agronomic practices. Making transgenic plants that can withstand biotic and abiotic challenges and altering them comes at a high expense. The development of PGPR formulations, including ACC deaminasethat assist plants in dealing with stressful conditions, has become the focus ofaddressing this problem.
CONCLUSION
By 2050, there will be roughly 9.7 billion people on the planet, which means that 50% more food will be needed than in the current situation. Consequently, it is critical to increase agricultural output, alleviate the harmful effects of environmental stresses, and provide sustainable food for the world's population. Utilizing bacteria that promote plant growth is an effective method to improve theyields of crops in harsh conditions and mitigate the negative impacts of environmental stresses. These bacteria play a significant role in the growth and development of plants. The rhizospheric bacteria that produce ACC deaminase have an obvious advantage over PGPRs lacking ACC deaminase because they protect the plants from the detrimental impacts of ethylene stress in stressful conditions. In conclusion, it would be highly recommended to isolate ACC deaminase-producing bacteria from various biological places, investigate their function in reducing plant stress, and encourage using ACC deaminase PGP bacteria instead of chemicals used in agricultural systems.
AUTHOR CONTRIBUTION
All authors listed have contributed substantially, directly, and intellectually to the work and approved the manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest associated with the manuscript.
ETHICAL APPROVAL
This article contains no studies with human participants or animals performed by authors.
ACKNOWLEDGMENTS
We extend our appreciation to Researchers Supporting Project No. RSP2024R218, King Saud University, Riyadh, Saudi Arabia, for partly funding this research work.
REFERENCES
- Ali, S., Khan, M. A., & Kim, W.-C. (2018). Pseudomonas veronii KJ mitigates flood stress-associated damage in Sesamum indicum L. Applied Biological Chemistry, 61(5), 575-585.
- Ali, S., & Kim, W.-C. (2018). Plant growth promotion under water: decrease of waterlogging-induced ACC and ethylene levels by ACC deaminase-producing bacteria. Frontiers in microbiology, 9, 1096.
- Ali, S., Moon, Y.-S., Hamayun, M., Khan, M. A., Bibi, K., & Lee, I.-J. (2022). Pragmatic role of microbial plant biostimulants in abiotic stress relief in crop plants. Journal of Plant Interactions, 17(1), 705-718.
- Cedeño-García, G. A., Gerding, M., Moraga, G., Inostroza, L., Fischer, S., Sepúlveda-Caamaño, M., & Oyarzúa, P. (2018). Plant growth promoting rhizobacteria with ACC deaminase activity isolated from Mediterranean dryland areas in Chile: Effects on early nodulation in alfalfa. Chilean journal of agricultural research, 78(3), 360-369.
- Chieb, M., & Gachomo, E. W. (2023). The role of plant growth promoting rhizobacteria in plant drought stress responses.BMC Plant Biology, 23(1), 407.
- Glick, B. R. (2014). Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiological research, 169(1), 30-39.
- Goswami, D., Thakker, J. N., & Dhandhukia, P. C. (2016). Portraying mechanics of plant growth promoting rhizobacteria (PGPR): A review. Cogent Food & Agriculture, 2(1), 1127500.
- Gupta, S., & Pandey, S. (2019). ACC deaminase producing bacteria with multifarious plant growth promoting traits alleviates salinity stress in French bean (Phaseolus vulgaris) plants. Frontiers in microbiology, 10, 1506.
- Jain, R., & Saraf, M. (2023). ACC deaminase producing PGPR modulates nutrients uptake, soil properties and growth of cluster bean (Cyamopsis tetragonoloba L.) under deficit irrigation. Biologia, 1-14.
- Kang, B. G., Kim, W. T., Yun, H. S., & Chang, S. C. (2010). Use of plant growth-promoting rhizobacteria to control stress responses of plant roots. Plant Biotechnology Reports, 4, 179-183.
- Khan, M. A., Asaf, S., Khan, A. L., Adhikari, A., Jan, R., Ali, S., . . . Lee, I.-J. (2019). Halotolerant rhizobacterial strains mitigate the adverse effects of NaCl stress in soybean seedlings. BioMed research international, 2019.
- Mantri, N., Patade, V., Penna, S., Ford, R., & Pang, E. (2012). Abiotic stress responses in plants: present and future. Abiotic stress responses in plants: metabolism, productivity and sustainability, 1-19.
- Mellidou, I., & Karamanoli, K. (2022). Unlocking PGPR-mediated abiotic stress tolerance: What lies beneath. Frontiers in Sustainable Food Systems, 6.
- Moon, Y.-S., & Ali, S. (2022a). A fruitful decade of bacterial ACC deaminase biotechnology: a pragmatic approach towards abiotic stress relief in plants. Theoretical and Experimental Plant Physiology, 1-21.
- Moon, Y.-S., & Ali, S. (2022b). Isolation and identification of multi-trait plant growth–promoting rhizobacteria from coastal sand dune plant species of Pohang beach. Folia Microbiologica, 67(3), 523-533.
- Moon, Y. S., & Ali, S. (2022). Possible mechanisms for the equilibrium of ACC and role of ACC deaminase-producing bacteria. Applied microbiology and biotechnology, 106(3), 877-887.
- Murali, M., Gowtham, H. G., Singh, S. B., Shilpa, N., Aiyaz, M., Niranjana, S. R., & Amruthesh, K. (2021). Bio-prospecting of ACC deaminase producing Rhizobacteria towards sustainable agriculture: A special emphasis on abiotic stress in plants. Applied Soil Ecology, 168, 104142.
- Naing, A. H., Maung, T. T., & Kim, C. K. (2021). The ACC deaminase‐producing plant growth‐promoting bacteria: influences of bacterial strains and ACC deaminase activities in plant tolerance to abiotic stress. Physiologia Plantarum, 173(4), 1992-2012.
- Nascimento, F., Brígido, C., Alho, L., Glick, B. R., & Oliveira, S. (2012). Enhanced chickpea growth-promotion ability of a Mesorhizobium strain expressing an exogenous ACC deaminase gene. Plant and Soil, 353, 221-230.
- Penrose, D. M., & Glick, B. R. (2003). Methods for isolating and characterizing ACC deaminase‐containing plant growth‐promoting rhizobacteria. Physiologia Plantarum, 118(1), 10-15.
- Qureshi, H., Abbas, M. H., Jan, T., Mumtaz, K., Mukhtar, H., & Khan, U. (2022). Plant Responses to Heat Stress. Journal Advances of Nutrition Science & Technology (ANST), 2(2).
- Qureshi, H., & Ahmed, W. (2022). Role of Plant Hormones Under Abiotic Stress Conditions. Journal Advances of Nutrition Science & Technology (ANST), 2(1).
- Santoyo, G., Urtis-Flores, C. A., Loeza-Lara, P. D., Orozco-Mosqueda, M. d. C., & Glick, B. R. (2021). Rhizosphere colonization determinants by plant growth-promoting rhizobacteria (PGPR). Biology, 10(6), 475.
- Shahid, M., Singh, U. B., Khan, M. S., Singh, P., Kumar, R., Singh, R. N., . . . Singh, H. V. (2023). Bacterial ACC deaminase: Insights into enzymology, biochemistry, genetics, and potential role in amelioration of environmental stress in crop plants. Frontiers in microbiology, 14, 1132770.
- Singh, S., Chanotiya, C. S., Singh, A., Vajpayee, P., & Kalra, A. (2023). Role of ACC-deaminase synthesizing Trichoderma harzianum and plant growth-promoting bacteria in reducing salt-stress in Ocimum sanctum. Physiology and Molecular Biology of Plants, 1-14.
- Waheed, A., Hamid, N., & Aagha, F. (2023). Multi-omic Techniques and Nitrogen (N) Induced Drought Tolerance in Rice Crop. Journal Advances of Nutrition Science & Technology (ANST), 3(1).
- Walker, T. S., Bais, H. P., Grotewold, E., & Vivanco, J. M. (2003). Root exudation and rhizosphere biology. Plant physiology, 132(1), 44-51.
How to Cite this paper?
APA-7 Style
Ali,
S., Ahmad,
M., Hamayun,
M., Alrefaei,
A.F. (2023). Rhizobacteria Containing ACC Deaminase Enhance Plant Growth and Tolerance Under Abiotic Stress Conditions. Journal Advances of Nutrition Science and Technology, 3(3-4), 72-77. https://doi.org/10.15228/ANST.2023.v03.i03-4.p08
ACS Style
Ali,
S.; Ahmad,
M.; Hamayun,
M.; Alrefaei,
A.F. Rhizobacteria Containing ACC Deaminase Enhance Plant Growth and Tolerance Under Abiotic Stress Conditions. J. Adv. Nutri. Sci. Tech. 2023, 3, 72-77. https://doi.org/10.15228/ANST.2023.v03.i03-4.p08
AMA Style
Ali
S, Ahmad
M, Hamayun
M, Alrefaei
AF. Rhizobacteria Containing ACC Deaminase Enhance Plant Growth and Tolerance Under Abiotic Stress Conditions. Journal Advances of Nutrition Science and Technology. 2023; 3(3-4): 72-77. https://doi.org/10.15228/ANST.2023.v03.i03-4.p08
Chicago/Turabian Style
Ali, Sajid, Mushtaq Ahmad, Muhammad Hamayun, and Abdulwahed Fahad Alrefaei.
2023. "Rhizobacteria Containing ACC Deaminase Enhance Plant Growth and Tolerance Under Abiotic Stress Conditions" Journal Advances of Nutrition Science and Technology 3, no. 3-4: 72-77. https://doi.org/10.15228/ANST.2023.v03.i03-4.p08

This work is licensed under a Creative Commons Attribution 4.0 International License.

